LILRA5 Functions to Induce ROS Production on Innate Immune Cells
- PMID: 41116296
- PMCID: PMC12537994
- DOI: 10.1002/eji.70079
LILRA5 Functions to Induce ROS Production on Innate Immune Cells
Abstract
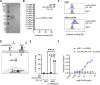
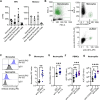
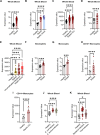
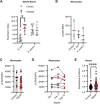
Activating immune receptors provides mechanisms for phagocytes to elicit important effector functions that promote the killing of microbes. Leukocyte immunoglobulin-like receptor A5 (LILRA5), an orphan immune receptor expressed by human phagocytes and co-localising with FcRγ, remains poorly characterised. To address this, we developed a highly specific anti-LILRA5 monoclonal antibody that has agonistic properties. We show LILRA5 expression on naïve monocytes and neutrophils, and that ligation of LILRA5 stimulates ROS production. While increased LILRA5 transcripts have been associated with sepsis, we also observed increased levels in patients with systemic infection but without sepsis complications. Ex vivo bacterial infection of whole blood did not alter surface LILRA5 expression, but LPS stimulation changed expression levels, indicating that surface LILRA5 expression is dynamic and likely regulated post-transcriptionally, changing responses to different stimuli or over time. Soluble (s)LILRA5 was enhanced in sera from sepsis patients and in supernatants of monocytes that were LPS-stimulated, indicating that shedding of LILRA5 from cell surfaces or that expression of sLILRA5 isoforms provides a mechanism to regulate surface LILRA5 expression levels. Finally, we show that altered surface LILRA5 expression influences LILRA5-induced ROS production capacity. Thus, LILRA5 is a dynamically regulated activating receptor expressed on phagocytes that stimulates ROS production.
Keywords: LILRA5; activating receptor; infection; reactive oxygen species; sepsis.
© 2025 The Author(s). European Journal of Immunology published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Dale D. C., Boxer L., and Liles W. C., “The Phagocytes: Neutrophils and Monocytes,” Blood 112 (2008): 935–945. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

