Long-term effectiveness of thymectomy in late-onset myasthenia gravis
- PMID: 41118002
- PMCID: PMC12540559
- DOI: 10.1007/s00415-025-13424-2
Long-term effectiveness of thymectomy in late-onset myasthenia gravis
Abstract
Background: Thymectomy is a well-established treatment in anti-AChR generalized myasthenia gravis (gMG) patients aged 18-50 years. However, the MGTX trial failed to prove an additional benefit of thymectomy in late-onset MG (LOMG) patients and studies conducted so far have shown controversial results. The primary aim of this study was to assess the safety and effectiveness of thymectomy in LOMG patients compared to medical therapy alone.
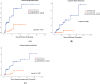
Methods: This was an observational retrospective case-control study of LOMG patients followed at the MG Clinic of Pisa University Hospital from 1996 to 2024. Inclusion criteria were: diagnosis of non-thymomatous gMG with anti-AChR antibodies; age at onset ≥ 50 years, and minimum follow-up of 12 months. The cumulative incidence of disease remission between the thymectomy and the conservative group was compared with Kaplan-Meier analysis with log-rank test and the Cox regression model was used to estimate the effect of thymectomy on achieving remission after adjustment for confounding variables.
Results: Among our population of 127 LOMG patients, 87 patients underwent thymectomy, while 40 patients received medical treatment only. When evaluating neurological outcomes at the last follow-up, the thymectomy group had a 3.25-fold (HR = 3.25, 95% CI 1.31-8.1) increased probability of achieving disease remission than the conservative group, after adjustment for confounding variables.
Conclusion: Our findings suggest that thymectomy may be a feasible and potentially beneficial therapeutic option in this MG subgroup, possibly increasing the likelihood of disease remission without ongoing immunosuppressive therapy.
Keywords: Anti-AChR Ab; Generalized myasthenia gravis; Late onset myasthenia gravis; Myasthenia gravis; Thymectomy; Thymic atrophy; Thymic hyperplasia; Very late onset myasthenia gravis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflicts of interest: MG received personal fees from Alexion, UCB, Argenx and Amgen outside the submitted work. GSreceived personal fees from Biogen, Roche, Sanofi, Lupin, Sarepta, Amicus, Csl Behring, Takeda, Grifols,Alnylam outside the submitted work. CCZ are official proctors for Intuitive Surgical. MM received lectureshonoraria from Alexion and personal fees from Alexion, Argenx, UCB, Johnson & Johnson, Idorsia outsidethe submitted work.EL, AC, SR, GR, RR have nothing to disclose. Ethical approval: Ethical approval was granted by Local Ethical Committee CEAVNO (Application ID: 19211) and the study was conducted in accordance with the Declaration of Helsinki and its amendments.
Figures


References
-
- Gilhus NE, Verschuuren JJ (2015) Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol 14(10):1023–1036. 10.1016/S1474-4422(15)00145-3 - PubMed
-
- Aarli JA (2008) Myasthenia gravis in the elderly: is it different? Ann N Y Acad Sci 1132(1):238–243. 10.1196/annals.1405.040 - PubMed
-
- Živković SA, Clemens PR, Lacomis D (2012) Characteristics of late-onset myasthenia gravis. J Neurol 259(10):2167–2171. 10.1007/s00415-012-6478-6 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

