Preserved Cognitive Function After Statin Administration During Cancer Treatment With Doxorubicin: A Secondary Analysis of a Randomized Clinical Trial
- PMID: 41118164
- PMCID: PMC12541534
- DOI: 10.1001/jamanetworkopen.2025.38325
Preserved Cognitive Function After Statin Administration During Cancer Treatment With Doxorubicin: A Secondary Analysis of a Randomized Clinical Trial
Abstract
Importance: Receipt of anthracycline-based chemotherapy and statin therapy have been found to be associated with deterioration of cognitive function in patients with cancer.
Objective: To assess the association of statins with attention, verbal fluency, and executive function in patients receiving doxorubicin treatment for cancer.
Design, setting, and participants: This secondary analysis of the Preventing Anthracycline Cardiovascular Toxicity With Statins randomized clinical trial, conducted across 31 US community and academic sites from February 5, 2014, through September 24, 2020, involved participants with stage I to IV lymphoma or stage I to III breast cancer undergoing doxorubicin treatment. The data analysis was conducted between August 1, 2024, and April 4, 2025.
Interventions: Participants were randomized (1:1) to receive a daily 40-mg dose of atorvastatin or placebo before initiating doxorubicin treatment and then continued for 24 months.
Main outcomes and measures: Attention, executive function, and verbal fluency were assessed using the Trail Making Test part A (TMT-A) and part B (TMT-B) and the Controlled Oral Word Association test, respectively. Time to complete the assessments were modeled using a linear mixed model, while errors (counts) were modeled using a generalized linear mixed model assuming a Poisson distribution.
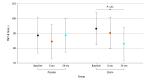
Results: A total of 238 participants (mean [SD] age, 49 [12] years; 217 female [91.2%]) were randomized to the statin (n = 118) or placebo (n = 120) groups. Values for TMT-A at 6 and 24 months were similar between the statin group (mean, 32.5 seconds [95% CI, 29.4-35.7 seconds] and 29.8 seconds [95% CI, 26.4-33.2 seconds], respectively) and the placebo group (mean, 28.4 seconds [95% CI, 25.2-31.5 seconds] and 27.8 seconds [95% CI, 24.5-31.0 seconds], respectively). From before treatment to 24 months after treatment, statin recipients had a significant mean improvement of 10.2 seconds for TMT-B (95% CI, 1.9-18.5 seconds), whereas placebo recipients had a nonsignificant improvement of only 0.2 seconds for TMT-B (95% CI, -8.5 to 8.1 seconds). Analysis of the time-by-treatment interaction showed no significant difference between groups. For Controlled Oral Word Association scores in the same period, both the placebo and statin groups showed a mean improvement of 3.62 points (95% CI, 1.71-5.54 points) and 4.74 points (95% CI, 2.69-6.79 points), respectively, with no difference between groups.
Conclusions and relevance: This secondary analysis of a randomized clinical trial suggests that the receipt of statins during doxorubicin therapy for lymphoma or breast cancer was not associated with worsening cognitive functions over 24 months. Within-group analyses suggested that statins may have contributed to better executive function on the TMT-B.
Trial registration: ClinicalTrials.gov Identifier: NCT01988571.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

