Implantation of a vascular access button in mice
- PMID: 41120542
- PMCID: PMC12541028
- DOI: 10.1038/s41598-025-20542-4
Implantation of a vascular access button in mice
Abstract
Vascular access presents unique challenges in experimental mice due to their small size and anatomical constraints. Achieving reliable vascular access is crucial for optimizing experimental outcomes, especially in protocols requiring serial blood sampling or repeated intravascular therapy. Although vascular access buttons (VABs) offer significant advantages, their widespread adoption has been limited by technical challenges and a lack of comprehensive validation regarding their safety, feasibility, and long-term management. To address these gaps, we conducted a comprehensive evaluation of VAB implantation in mice. The technical success rate was 90.2%, and the 28-day survival rate was 80.4%. Optimal catheter insertion lengths determined by intraoperative and autopsy findings, and computed tomography were 9.5 ± 0.6 mm (20-25 g), 10.1 ± 0.8 mm (25-30 g), and 11.2 ± 0.5 mm (> 30 g), respectively. Catheter patency was analyzed by stratifying the cohort into groups based on physical parameters such as catheter tip geometry, heparin concentration in the maintenance solution, and frequency of catheter maintenance procedures. Although approximately half of the mice lost complete catheter patency by day 14, the majority maintained partial patency at day 28 in all experimental groups except for two cases of complete occlusion in the 2 Fr square tip, low-dose heparin, weekly maintenance group. The evaluation of biodistribution and clearance with indocyanine green indicated that VAB administration may have advantages over conventional venipuncture. These standardized methodologies can provide a framework for diverse biomedical research applications, enhancing both the efficiency and reproducibility of studies requiring reliable vascular access.
Keywords: External jugular vein; Vascular access; Vascular access button; Vascular catheter.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures
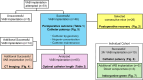


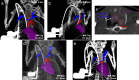



References
-
- Waterston, R. H. et al. Initial sequencing and comparative analysis of the mouse genome. Nature420, 520–562. 10.1038/nature01262 (2002). - PubMed
-
- Burvin, R., Zloczower, M. & Karnieli, E. Double-vein jugular/inferior vena cava clamp technique for long-term in vivo studies in rats. Physiol. Behav.63, 511–515. 10.1016/s0031-9384(97)00486-1 (1998). - PubMed
-
- Noble, F., Coric, P., Turcaud, S., Fournié-Zaluski, M. C. & Roques, B. P. Assessment of physical dependence after continuous perfusion into the rat jugular vein of the mixed inhibitor of enkephalin-degrading enzymes, RB 101. Eur. J. Pharmacol.253, 283–287. 10.1016/0014-2999(94)90203-8 (1994). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

