Impact of diastolic blood pressure time under range on mortality and acute kidney injury in septic patients: a retrospective cohort study
- PMID: 41120869
- PMCID: PMC12538847
- DOI: 10.1186/s12871-025-03382-7
Impact of diastolic blood pressure time under range on mortality and acute kidney injury in septic patients: a retrospective cohort study
Abstract
Background: Current guidelines for sepsis management focus on maintaining mean arterial pressure, while the impact of low diastolic blood pressure (DBP) exposure remains unclear. This study investigated whether the time under range of DBP (DBP-TUR) is associated with clinical outcomes in septic patients who achieved conventional blood pressure targets.
Methods: In this retrospective cohort study using the MIMIC-IV database, we included 12,114 adult patients with sepsis. DBP-TUR was defined as the proportion of time with DBP < 50 mmHg while maintaining systolic blood pressure > 90 mmHg or mean arterial pressure > 65 mmHg during the first 48 h after ICU admission. Primary outcome was 28-day mortality, and secondary outcome was acute kidney injury (AKI).
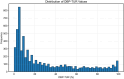
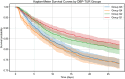
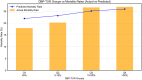
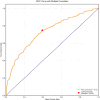
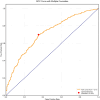
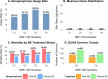
Results: Among the cohort, 6,192 patients (51.1%) experienced low DBP exposure. Patients were stratified into quartiles based on DBP-TUR (Q1: ≤5%, Q2: 5-15%, Q3: 15-50%, Q4: >50%). After adjusting for confounders, compared with Q1, both Q3 (OR:1.25, 95% CI:1.02-1.54) and Q4 (OR:1.27, 95% CI:1.02-1.57) showed significantly higher 28-day mortality. Similarly, AKI risk increased in Q3 (OR:1.47, 95% CI:1.14-1.91) and Q4 (OR:1.60, 95% CI:1.20-2.14). DBP-TUR demonstrated moderate predictive value for both mortality (AUC:0.73) and AKI (AUC:0.71).
Conclusion: Low DBP exposure, despite achieving conventional blood pressure targets, was independently associated with increased mortality and AKI risk in septic patients. Monitoring DBP-TUR might provide additional value in hemodynamic management of sepsis.
Keywords: Acute kidney injury; Diastolic blood pressure; Mortality; Sepsis; Time under range.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The MIMIC-IV database was approved by the Institutional Review Boards of Massachusetts Institute of Technology (Cambridge, MA) and Beth Israel Deaconess Medical Center (Boston, MA). Informed consent was obtained for the original data collection. The requirement for individual patient consent was waived as the project did not impact clinical care and all protected health information was de-identified. Not applicable. This study utilized the publicly available de-identified MIMIC-IV database which received ethical approval including waiver of informed consent by the Institutional Review Boards of Beth Israel Deaconess Medical Center (BIDMC) and the Massachusetts Institute of Technology (MIT). Consent for publication: Not applicable. This study utilized the publicly available de-identified MIMIC-IV database. Competing interests: The authors declare no competing interests.
Figures
References
-
- Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585. 10.1136/bmj.i1585. - PubMed
-
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43:304–77. 10.1007/s00134-017-4683-6. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

