5-Amino- and 6-amino-uracils in the synthesis of various heterocycles as therapeutic agents: a review
- PMID: 41122147
- PMCID: PMC12536882
- DOI: 10.1039/d5ra05123a
5-Amino- and 6-amino-uracils in the synthesis of various heterocycles as therapeutic agents: a review
Abstract
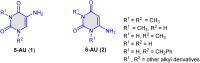
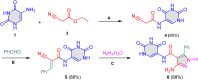
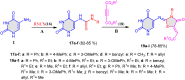
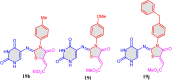
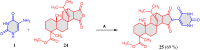
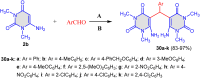
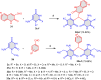
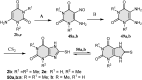
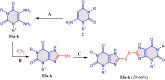
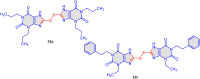
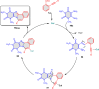
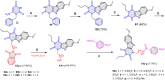
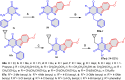
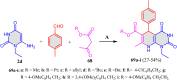
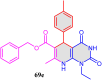
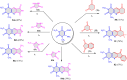
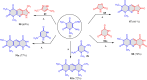
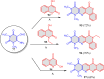
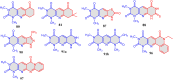
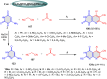
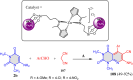
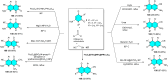
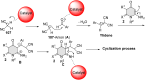
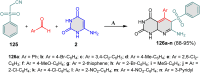
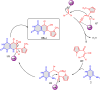
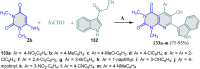
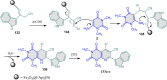
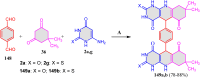
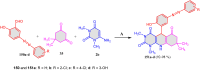
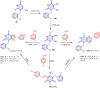
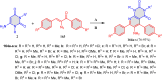
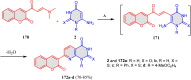
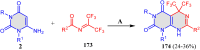
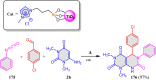
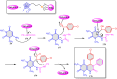
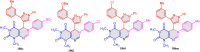
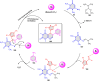
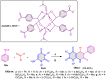
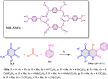
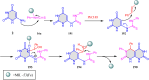
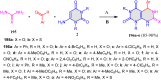
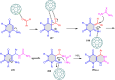
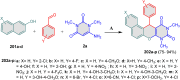
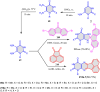
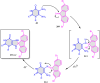
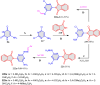
5-Amino- and 6-amino-uracil derivatives are used as precursors in the synthesis of various heterocyclic compounds, which have attracted great interest because of their potent biological activities and therapeutic uses. Multicomponent reactions (MCRs) are a quite significant green technique as they directly correlate with fewer byproducts as well as lower time and energy consumption. These benefits of MCRs expand their potential in the preparation of a variety of new catalytic systems for the synthesis of essential organic compounds in environmentally friendly reaction circumstances. Herein, we focus on some MCR sequences that evolved during the last decade, especially from 2014 to 2024, for the synthesis of target heterocycles starting from either 5-amino- or 6-amino-uracil derivatives. In addition, we discuss the mechanism by which the selected catalyst helps in the selectivity of the target molecules. Furthermore, the biological activity of the synthesized materials as therapeutic agents was reviewed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










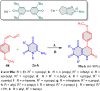

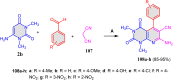


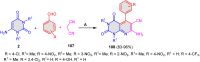
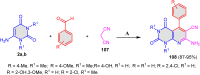
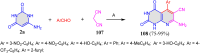

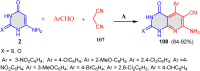
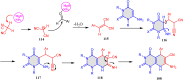
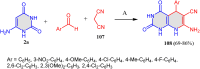



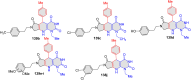
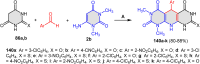
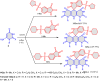

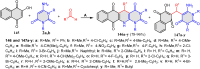














References
-
- Patrick G. L., An Introduction to Medicinal Chemistry, Oxford university press, 2023
-
- Brown D. J., Evans R. F., Cowden W. B., Fenn M. D., Chemistry of Heterocyclic Compounds: The Pyrimidines, 1994, vol. 52, 10.1002/9780470187395 - DOI
Publication types
LinkOut - more resources
Full Text Sources

