Blood pressure variability compromises vascular function in middle-aged mice
- PMID: 41123590
- PMCID: PMC12543325
- DOI: 10.7554/eLife.104082
Blood pressure variability compromises vascular function in middle-aged mice
Abstract
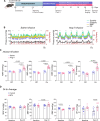
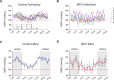
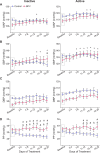
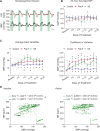
Blood pressure variability (BPV) has emerged as a significant risk factor for cognitive decline and dementia, independent of alterations in average blood pressure (BP). However, the impact of large BP fluctuations on neurovascular function remains poorly understood. In this study, we developed a novel murine model of BPV in middle-aged mice using intermittent angiotensin II infusions. Radio telemetry confirmed that 24 hr BP averages in BPV mice remained comparable to controls, demonstrating BPV in the absence of hypertension. Chronic (20-25 days) BPV resulted in a blunted bradycardic response and cognitive deficits. Two-photon imaging revealed heightened pressure-evoked constrictions (myogenic response) in parenchymal arterioles of BPV mice. While sensory stimulus-evoked dilations (neurovascular coupling) were amplified at higher BP levels in control mice, this pressure-dependent effect was abolished in BPV mice. Our findings indicate that chronic BP fluctuations impair vascular function within the neurovascular complex and contribute to cognitive decline, emphasizing BPV as a critical factor in brain health.
Keywords: autoregulation; blood pressure; bradycardic; cognitive; mouse; myogenic; neuroscience; neurovascular.
© 2025, Mendiola et al.
Conflict of interest statement
PM, PO, KX, MB, WB, RP, VD, JF No competing interests declared
Figures



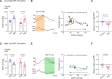


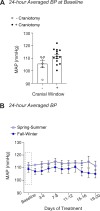
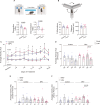
Update of
-
Blood pressure variability compromises vascular function in middle-aged mice.bioRxiv [Preprint]. 2025 Jul 2:2024.10.21.619509. doi: 10.1101/2024.10.21.619509. bioRxiv. 2025. Update in: Elife. 2025 Oct 22;14:RP104082. doi: 10.7554/eLife.104082. PMID: 39484398 Free PMC article. Updated. Preprint.
References
-
- Benfato ID, Quintanilha ACS, Henrique JS, Souza MA, Rosário BDA, Beserra Filho JIA, Santos RLO, Ribeiro AM, Le Sueur Maluf L, de Oliveira CAM. Effects of long-term social isolation on central, behavioural and metabolic parameters in middle-aged mice. Behavioural Brain Research. 2022;417:113630. doi: 10.1016/j.bbr.2021.113630. - DOI - PMC - PubMed
-
- Böhm M, Schumacher H, Leong D, Mancia G, Unger T, Schmieder R, Custodis F, Diener HC, Laufs U, Lonn E, Sliwa K, Teo K, Fagard R, Redon J, Sleight P, Anderson C, O’Donnell M, Yusuf S. Systolic blood pressure variation and mean heart rate is associated with cognitive dysfunction in patients with high cardiovascular risk. Hypertension. 2015;65:651–661. doi: 10.1161/HYPERTENSIONAHA.114.04568. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

