Neuromuscular blockade and their monitoring in the intensive care unit: a multicenter observational prospective study
- PMID: 41123780
- PMCID: PMC12546236
- DOI: 10.1186/s13613-025-01591-4
Neuromuscular blockade and their monitoring in the intensive care unit: a multicenter observational prospective study
Abstract
Background: Neuromuscular blocking agents may improve outcomes in specific conditions, including the early phase of acute respiratory distress syndrome. However, neuromuscular blocking agents are associated with side effects and uncertainty persists regarding their optimal dosing and efficacy. Our objective was to describe the use of neuromuscular blocking agents in a real-world setting.
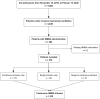
Methods: We conducted a multicenter, prospective observational study, including adult patients who underwent invasive mechanical ventilation and received a continuous infusion of neuromuscular blocking agents. Patients were recruited across 19 intensive care units in France and Belgium.
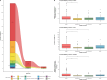
Results: From November 16, 2019, to February 19, 2020, a total of 2248 patients were hospitalized and mechanically ventilated in 19 participating ICUs. Of these, 270 (12%) patients received at least one dose of neuromuscular blocking agents, and 232 (10.3%) received a continuous infusion. The main indications for neuromuscular blocking agents use were acute respiratory distress syndrome (61%), prevention of shivering during therapeutic hypothermia (16%) and patient-ventilator asynchrony (12%). Infusion was initiated in median at 0 [0-2] days after ICU admission, with a median duration of 38 [22-71] hours. Cisatracurium was the preferred agent (74%). Neuromuscular blocking agents monitoring by train-of-four was employed in 48% of patients. Intensive care unit-acquired weakness was diagnosed in 25% of patients, pressure ulcers in 14% and ventilator-associated pneumonia in 26%. The median lengths of mechanical ventilation and ICU stay were 9 [4-16] and 13 [6-22] days, and ICU mortality was 41%. In multivariable analyses, a duration of neuromuscular blocking agents infusion exceeding 48 hours was associated with a lower cumulative incidence of weaning success (SHR 0.83 [0.76, 0.91], p < 0.001) and higher incidences of ventilator-associated pneumonia, while neuromuscular blocking agents monitoring was associated with both increased intensive care unit-acquired weakness (OR 2.90 [1.2, 7.01], p = 0.018) and reduced ICU mortality (HR 0.55 [95%CI 0.32, 0.95], p = 0.032).
Conclusion: In our study, the prevalence of continuous neuromuscular blocking agents infusion among mechanically ventilated patients in the intensive care unit was 10.3%. While acute respiratory distress syndrome was the main indication, over one-third of patients received neuromuscular blocking agents for other reasons. A duration of neuromuscular blocking agents infusion exceeding 48 hours was associated with longer mechanical ventilation and increased complications. The role of neuromuscular blocking agents monitoring remains unclear. Trial registration ClinicalTrials.gov: NCT04028362 Registered on 18 July 2019, https://clinicaltrials.gov/study/NCT04028362 . The study was conducted by the French Intensive Care Society/Société de Réanimation de Langue Française Trial Group.
Keywords: Acute respiratory distress syndrome; Critical care; Mechanical ventilation; Neuromuscular blockade.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study was approved by the Comité de Protection des Personnes Sud-Ouest et Outre-Mer 2 n°ID-RCB 2019-A01378-49 and was prospectively registered in ClinicalTrials.gov (NCT04028362). Informed consent was obtained from patients’ relatives. Reporting adhered to the STROBE guidelines. Consent for publication: Not applicable Competing interests: The authors declare no competing interest.
Figures

References
-
- Lascarrou JB, Le Gouge A, Dimet J, Lacherade JC, Martin-Lefevre L, Fiancette M, et al. Neuromuscular blockade during therapeutic hypothermia after cardiac arrest: observational study of neurological and infectious outcomes. Resuscitation. 2014;85(9):1257–62. - PubMed
-
- Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical

