In silico nephroprotective evaluation of microbial biotransformed metabolites from Aframomum melegueta
- PMID: 41123816
- PMCID: PMC12546235
- DOI: 10.1186/s13568-025-01962-x
In silico nephroprotective evaluation of microbial biotransformed metabolites from Aframomum melegueta
Abstract
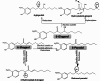
Microbial biotransformation of three bioactive phenolic constituents from Aframomum melegueta K. schum, namely 6-gingerol, 6-paradol and 6-shogaol, was performed by Bacillus subtilis 168, Pseudomonas aeruginosa PO1A and Candida albicans ATCC10231. Structures of the isolated compounds were determined using LC/MS analyses. To assess and compare their potential nephroprotective effects, the parent compounds and their biotransformation metabolites were subjected to molecular docking studies targeting AMP-activated protein kinase (AMPK) for the first time. During microbial biotransformation, a series of reactions, primarily hydroxylation and reduction, were observed, resulting in the identification of five distinct metabolites. LC/MS analysis of the fermentation medium revealed that Bacillus subtilis 168 converted 6-gingerol into 6-gingerdiol (M1) and hydroxylated 6-gingerol (M2), while 6-shogaol was transformed into 6-paradol (M3) and hydroxylated 6-shogaol (M4). Additionally, 6-paradol underwent further reduction to form (M5). Docking results showed that all compounds demonstrated binding affinity to AMPK, indicating potential nephroprotective activity. Notably, M1 exhibited the highest binding affinity, suggesting its strong therapeutic promise as a nephroprotective agent of natural origin. M5 ranked second in binding affinity, followed by M4. These results highlight the effectiveness of microbial transformation in generating bioactive derivatives with potentially enhanced biological activity compared to their natural precursors.
Supplementary Information: The online version contains supplementary material available at 10.1186/s13568-025-01962-x.
Keywords: Aframomum melegueta; Biotransformation; Gingerol; Nephroprotective activity; Paradol; Shogaol.
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
-
- Abdou RM, El-Maadawy WH, Hassan M, El-Dine RS, Aboushousha T, El-Tanbouly ND, El-Sayed AM (2021) Nephroprotective activitiy of Aframomum melegueta seeds extract against diclofenac-induced acute kidney injury: a mechanistic study. J Ethnopharmacol. 10.1016/j.jep.2021.113939 - PubMed
-
- Alfarra HY, Omar MN (2012) Microbial transformation of natural products. Greener J Biol Sci 3(10):357–364
-
- Cichewicz RH, Kouzi SA (1998) Biotransformation of resveratrol to piceid by Bacillus cereus. J Nat Prod 61(10):1313–1314. 10.1021/np980139b - PubMed
-
- Dugasani S, Pichika MR, Nadarajah VD, Balijepalli MK, Tandra S, Korlakunta JN (2010) Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J Ethnopharmacol 3(2):515–520. 10.1016/j.jep.2009.10.004 - PubMed
LinkOut - more resources
Full Text Sources

