External factors influence intrinsic differences in Stx2e production by Porcine Shiga Toxin-producing Escherichia coli strains
- PMID: 41124219
- PMCID: PMC12571312
- DOI: 10.1371/journal.ppat.1013616
External factors influence intrinsic differences in Stx2e production by Porcine Shiga Toxin-producing Escherichia coli strains
Abstract
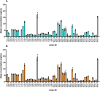
Porcine Shiga toxin-producing Escherichia coli (STEC) strains pose significant challenges to the pig industry. The toxins produced by these strains, particularly Shiga toxin subtype 2e (Stx2e), are associated with a range of clinical symptoms such as diarrhoea and oedema disease, which in severe cases result in death. Understanding the factors that influence the production and secretion of Stx2e is crucial to elucidate porcine STEC pathogenesis and to develop effective therapeutic strategies. Therefore, this study aimed to characterize the variability in Stx2e production among different porcine STEC strains and assess the effect of several external factors, including bile acids and antibiotics. Our results highlighted a substantial variation in extracellular Stx2e levels by porcine STEC strains. In addition, bile acids, especially the bile acid deoxycholate, exerted strain-specific effects on these extracellular Stx2e levels. Antibiotics also affected extracellular Stx2e levels with ciprofloxacin and enrofloxacin inducing a substantial increase in toxin production in certain strains. Genome analysis revealed that these strains encode a holin gene downstream of the Stx2e operon. Deleting this holin gene abolished the antibiotic-induced increase in extracellular Stx2e levels, while introducing holin expression in unresponsive strains increased the presence of Stx2e in the extracellular environment. These findings unravel a role for phage holins in Stx2e secretion and highlight the intricate interplay between genetic and environmental factors in regulating Stx2e production in porcine STEC strains. Together, our results offer insights into STEC pathogenesis.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
We have read the journal’s policy and the authors of this manuscript have the following competing interests: NV is a former employee at PathoSense BV. DS is employed at CEVA Animal Health.
Figures


References
-
- Jackson MP, Neill RJ, O’Brien AD, Holmes RK, Newland JW. Nucleotide sequence analysis and comparison of the structural genes for Shiga-like toxin I and Shiga-like toxin II encoded by bacteriophages fromEscherichia coli933. FEMS Microbiology Letters. 1987;44(1):109–14. doi: 10.1111/j.1574-6968.1987.tb02252.x - DOI
-
- Wang X, Yu D, Chui L, Zhou T, Feng Y, Cao Y, et al. A Comprehensive Review on Shiga Toxin Subtypes and Their Niche-Related Distribution Characteristics in Shiga-Toxin-Producing E. coli and Other Bacterial Hosts. Microorganisms. 2024;12(4):687. doi: 10.3390/microorganisms12040687 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

