Leptin as a key driver for organ fibrogenesis
- PMID: 41124259
- PMCID: PMC12542948
- DOI: 10.1126/sciadv.ady7904
Leptin as a key driver for organ fibrogenesis
Abstract
Leptin, a hormone primarily secreted by adipocytes, regulates energy balance and systemic metabolism through its interaction with the leptin receptor (LEPR). Beyond these functions, leptin signaling has been implicated in the pathogenesis of tissue fibrosis. Here, we report the x-ray crystal structures of a leptin-neutralizing antibody (hLep3) in the unbound and leptin-bound states. The interaction of this antibody with leptin mimics the interaction of the LEPR with leptin, providing direct insights into the mechanism by which the antibody disrupts leptin signaling. We furthermore evaluate the therapeutic potential of neutralizing leptin with this antibody across distinct mouse models of fibrosis affecting the kidney, liver, lung, heart, and blood vessels. Leptin neutralization markedly inhibited fibrosis progression in all models. Mechanistically, suppression of leptin activity reduces pro-inflammatory and profibrotic processes, underscoring its therapeutic potential. These findings suggest that leptin signaling plays a vital role in tissue fibrosis and that treatment with a leptin-neutralizing antibody may be a promising therapeutic approach.
Figures


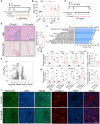
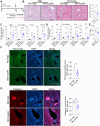

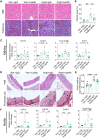
References
-
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M., Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994). - PubMed
-
- Caro J. F., Sinha M. K., Kolaczynski J. W., Zhang P. L., Considine R. V., Leptin: The tale of an obesity gene. Diabetes 45, 1455–1462 (1996). - PubMed
-
- Pan W. W., Myers M. G. Jr., Leptin and the maintenance of elevated body weight. Nat. Rev. Neurosci. 19, 95–105 (2018). - PubMed
-
- Barash I. A., Cheung C. C., Weigle D. S., Ren H., Kabigting E. B., Kuijper J. L., Clifton D. K., Steiner R. A., Leptin is a metabolic signal to the reproductive system. Endocrinology 137, 3144–3147 (1996). - PubMed
-
- Zhao S., Zhu Y., Schultz R. D., Li N., He Z., Zhang Z., Caron A., Zhu Q., Sun K., Xiong W., Deng H., Sun J., Deng Y., Kim M., Lee C. E., Gordillo R., Liu T., Odle A. K., Childs G. V., Zhang N., Kusminski C. M., Elmquist J. K., Williams K. W., An Z., Scherer P. E., Partial leptin reduction as an insulin sensitization and weight loss strategy. Cell Metab. 30, 706–719.e6 (2019). - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

