Renoprotective Effects of MIT-001 in Ischemia-Reperfusion Injury: Modulation of Ferroptosis, ROS and Fibrotic Markers
- PMID: 41126407
- PMCID: PMC12544698
- DOI: 10.1111/jcmm.70914
Renoprotective Effects of MIT-001 in Ischemia-Reperfusion Injury: Modulation of Ferroptosis, ROS and Fibrotic Markers
Abstract
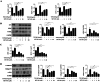
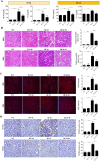
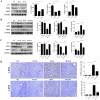
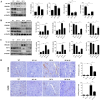
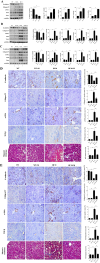
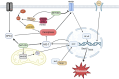
Renal ischemia-reperfusion injury (IRI) is a key driver of the progression from acute kidney injury (AKI) to chronic kidney disease (CKD), primarily through mechanisms involving oxidative stress, ferroptosis, and inflammation that promote fibrotic remodelling. This study investigates the therapeutic potential of MIT-001, a mitochondria-targeted reactive oxygen species (ROS) scavenger, in mitigating renal IRI. In vitro, MIT-001 attenuated ferroptotic cell death and fibrotic responses in HK-2 cells challenged with TGF-β or RSL3. MIT-001 restored GPX4 expression and activity, activated Nrf2 signalling, reduced lipid ROS and suppressed fibrogenic markers (α-SMA, Snail, collagen IV), while preserving E-cadherin levels. In a bilateral renal IRI mouse model, administration of MIT-001 significantly improved renal function and histology. Oxidative stress (DHE staining), apoptosis (TUNEL) and ferroptosis (4-HNE, xCT, GPX4) were markedly reduced. Additionally, MIT-001 inhibited the NF-κB/HMGB1 inflammatory axis and enhanced antioxidant defence via the Nrf2/HO-1 pathway, resulting in decreased immune infiltration and fibrosis. These findings demonstrate that MIT-001 confers renal protection by concurrently targeting oxidative stress, ferroptosis and inflammation, underscoring its promise as a therapeutic strategy to prevent AKI-to-CKD progression.
Keywords: MIT‐001; chronic kidney disease; ferroptosis; ischemia–reperfusion injury; oxidative stress; renal fibrosis.
© 2025 The Author(s). Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

