Chronic Sleep Fragmentation Differentially Affects Alzheimer's Disease Pathology in Male and Female APPSAA Knock-in Mice
- PMID: 41126970
- PMCID: PMC12537530
- DOI: 10.2147/JIR.S544625
Chronic Sleep Fragmentation Differentially Affects Alzheimer's Disease Pathology in Male and Female APPSAA Knock-in Mice
Abstract
Introduction: Sleep fragmentation often precedes Alzheimer's disease (AD) diagnosis and represents a potential modifiable risk factor, especially among women who have higher prevalence of both sleep disorders and AD.
Methods: This study investigated how chronic sleep fragmentation affects neuroinflammation and amyloid-beta (Aβ) accumulation in male and female APPSAA knock-in mice, a physiologically relevant AD model expressing APP at normal levels. APPSAA mice of both sexes (N=8/sex/strain, 8 months old) underwent either 5 weeks of chronic sleep fragmentation administered during the light phase using an automated sweeper system or undisturbed sleep. Sleep-wake patterns and circadian rhythms were monitored using piezoelectric sensors. Following intervention, we assessed neuroinflammatory markers via immunohistochemistry and multiplex cytokine analysis, Aβ levels in different solubility fractions, and Aβ plaque characteristics through digital pathology.
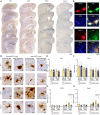
Results: Sleep fragmentation effectively disrupted sleep patterns in both sexes, reducing light-phase sleep and increasing intradaily variability. Sleep fragmentation increased GFAP immunoreactivity in both sexes, with larger effects in females than males. Surprisingly, sleep fragmentation decreased expression of the microglial activation markers MHCII and Dectin-1 in males. Pro-inflammatory cytokines (IL-1β, CCL2, CXCL2) were significantly elevated following sleep fragmentation, with distinct regional and sex-specific patterns. In females, sleep fragmentation increased PBS-soluble and formic acid-soluble Aβ in the neocortex and medium-sized plaque density in the hippocampus, while males showed decreased detergent-soluble Aβ in the neocortex following sleep fragmentation.
Discussion: Chronic sleep fragmentation exacerbates AD-related pathology in APPSAA mice in a sex-dependent manner, with females showing greater vulnerability to Aβ accumulation and astrocyte reactivity following sleep disruption. These findings suggest that environmental sleep disruptions may contribute to the higher prevalence of AD in women and highlight the importance of addressing sleep fragmentation as a modifiable risk factor for AD.
Keywords: amyloidosis; astrocyte reactivity; circadian rhythm; environmental stressors; neuroinflammation; sexual dimorphism.
© 2025 Hawkins et al.
Conflict of interest statement
BFO is a co-owner of Signal Solutions, LLC, which manufactures the piezoelectric sleep monitoring equipment used in this study. He also reports NIH grants to Signal Solutions, typically with 50% subawarded to UK, but not directly related to this manuscript (but potentially synergistic). On his current STTR award, he is the PI through his UK faculty position, even though the award is to Signal Solutions. He also reports multiple conflict of interest management plans, and a UK COI committee, to help him manage these potential conflicts of interest. He receives a portion of his yearly income from Signal Solutions, that is variable, but averages roughly 25% of his yearly salary. He attends most scientific meetings paid by Signal Solutions, even when presenting UK related research, mainly because it is easier; non-financial support includes admin support from his company, to free up his time for both UK and the company; reports patent “Stimulation System Based on Mechanical Vibration for Modification and Characterization of Sleep and Behavior in Rodents”, not directly relevant to the manuscript. MJD reports honoraria for reviews of grants related to research on Alzheimer’s disease from State of Florida Department of Health. All other authors report no conflicts of interest related to this work.
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous

