Nanocarrier-based immunotherapy for viral diseases
- PMID: 41127048
- PMCID: PMC12538933
- DOI: 10.1016/j.ijpx.2025.100408
Nanocarrier-based immunotherapy for viral diseases
Abstract
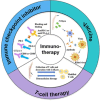
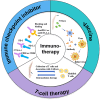
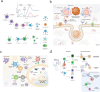
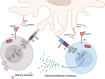
The global morbidity and mortality associated with viral diseases pose a major threat to public health security and cause significant economic losses worldwide. Developing novel prophylactic and therapeutic interventions remains an urgent priority in contemporary virology research. Immunotherapy, initially developed for cancer treatment, has shown satisfactory efficacy in the management of viral infections. However, the clinical application of immunotherapy is still constrained by its inherent limitations, including poor stability, inadequate targeting ability, and systemic toxicity. Nanocarriers have emerged as a promising platform to address these challenges, with features such as protecting active substances from enzymatic degradation, delivering active substances specifically to the site of infection via ligand modification, and controlling the release behavior of active substances so as to maintain their controlled and therapeutic concentrations. Therefore, the combination of immunotherapy and nanocarriers is expected to overcome the shortcomings of immunotherapy and significantly improve their therapeutic efficacy. In this review, the classification, application, and combination of immunotherapy with nanocarriers in viral diseases are summarized. The challenges and the future prospects of this combination are also discussed.
Keywords: Immune checkpoint inhibitors; Immunotherapy; Nanocarriers; T-cell immunotherapy; Vaccine; Viral diseases.
© 2025 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
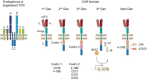
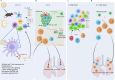

References
-
- Acúrcio R.C., Kleiner R., Vaskovich-Koubi D., Carreira B., Liubomirski Y., Palma C., Yeheskel A., Yeini E., Viana A.S., Ferreira V., Araújo C., Mor M., Freund N.T., Bacharach E., Gonçalves J., Toister-Achituv M., Fabregue M., Matthieu S., Guerry C., Zarubica A., Aviel-Ronen S., Florindo H.F., Satchi-Fainaro R. Intranasal multiepitope PD-L1-siRNA-based nanovaccine: the next-Gen COVID-19 immunotherapy. Adv. Sci. 2024;11 doi: 10.1002/advs.202404159. - DOI
-
- Adams D., Gonzalez-Duarte A., O’Riordan W.D., Yang C.C., Ueda M., Kristen A.V., Tournev I., Schmidt H.H., Coelho T., Berk J.L., Lin K.P., Vita G., Attarian S., Planté-Bordeneuve V., Mezei M.M., Campistol J.M., Buades J., Brannagan T.H., 3rd, Kim B.J., Oh J., Parman Y., Sekijima Y., Hawkins P.N., Solomon S.D., Polydefkis M., Dyck P.J., Gandhi P.J., Goyal S., Chen J., Strahs A.L., Nochur S.V., Sweetser M.T., Garg P.P., Vaishnaw A.K., Gollob J.A., Suhr O.B. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N. Engl. J. Med. 2018;379:11–21. doi: 10.1056/NEJMoa1716153. - DOI - PubMed
-
- Afraz S., Ghasemzadeh H., Dargahi M. Nanomagnetic hydrogel based on chitosan, hyaluronic acid, and glucose oxidase as a nanotheranostic platform for simultaneous therapeutic and imaging applications. Mater. Today Chem. 2022;24 doi: 10.1016/j.mtchem.2022.100807. - DOI
-
- Aminian F., Hemmati A. Dendrimers as versatile platforms for advanced biosensor development: a review on chemistry, synthesis, and performance enhancements. J. Environ. Chem. Eng. 2025;13 doi: 10.1016/j.jece.2025.116365. - DOI
Publication types
LinkOut - more resources
Full Text Sources

