Structural characteristics of a neutral Glycyrrhiza uralensis polysaccharide and its fermentation properties on the gut microbiota of immunocompromised rats in vivo and vitro
- PMID: 41127089
- PMCID: PMC12537741
- DOI: 10.3389/fnut.2025.1651015
Structural characteristics of a neutral Glycyrrhiza uralensis polysaccharide and its fermentation properties on the gut microbiota of immunocompromised rats in vivo and vitro
Abstract
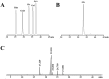
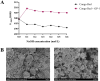
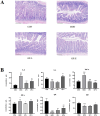
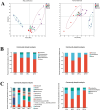
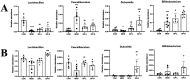
Glycyrrhiza polysaccharides (GPs) exhibit notable physiological activity; however, their structure is not well understood. The novel neutral polysaccharide fraction GP-1 was isolated from the roots and rhizomes of Glycyrrhiza uralensis Fisch. The fermentation properties of GP-1 were investigated in vitro and in vivo in immunocompromised rats. The molecular weight (Mw) of GP-1 is 7.6 kDa. The analysis of the monosaccharide composition indicated that GP-1 is a glucan. Nuclear magnetic resonance spectroscopy and methylation analyses revealed that GP-1 comprised a glucan main chain linked by α-D-Glcp-(1 → 4) bonds, with β-D-Glcp-(1 → 6)-α-D-Glcp-(1 → side branches at the C-6 position of 1,4,6-Glc and β-D-Glcp-(1 → side branches at the C-3 position of 1,3,4-Glc. Staining with Congo red confirmed the presence of a triple-helix structure. Scanning electron microscopy revealed that GP-1 has granular morphology. Pharmacological studies showed that GPs and GP-1 modulated the balance of the gut microbiota and influenced the production of short-chain fatty acids. In addition, the in vivo fermentation of GP and the in vitro fermentation of GP-1 promoted the growth of certain probiotics, particularly Lactobacillus and Dubosiella. These results underscore the structural characteristics of GP-1 and its potential as a prebiotic agent.
Keywords: Glycyrrhiza uralensis; fermentation; gut microbiota; neutral polysaccharide; structural characterisation.
Copyright © 2025 Sun, Wu, Li, Xie, Xiong, Jiang, Tang, Peng and Zheng.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Yang F, Chu T, Zhang Y, Liu X, Sun G, Chen Z. Quality assessment of licorice (Glycyrrhiza glabra L.) from different sources by multiple fingerprint profiles combined with quantitative analysis, antioxidant activity and chemometric methods. Food Chem. (2020) 324:126854. doi: 10.1016/j.foodchem.2020.126854, PMID: - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

