Autologous peripheral blood mononuclear cell-loaded injectable cell scaffold (ICS-001) for revolutionising angiogenic therapy: a study protocol for an exploratory clinical trial
- PMID: 41130688
- PMCID: PMC12551541
- DOI: 10.1136/bmjopen-2025-107486
Autologous peripheral blood mononuclear cell-loaded injectable cell scaffold (ICS-001) for revolutionising angiogenic therapy: a study protocol for an exploratory clinical trial
Abstract
Introduction: This research paper presents a study protocol for an exploratory clinical trial evaluating the safety and potential efficacy of autologous peripheral blood mononuclear cell (PBMNC)-loaded injectable cell scaffold (ICS-001) for angiogenic therapy in chronic limb-threatening ischaemia (CLTI). CLTI, the advanced stage of peripheral artery disease, presents significant therapeutic challenges.
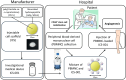
Methods and analysis: Angiogenic therapy using ICS-001 with PBMNCs-a novel approach designed to enhance local cell retention and promote neovascularisation-will be explored. The study will address the pathophysiology of CLTI, the limitations of current treatments and the rationale for cell-based therapies, alongside the clinical trial design for evaluating the safety and efficacy of ICS-001. We hypothesise that ICS-001 will improve ulcer healing and reduce ischaemic rest pain in patients with CLTI. This paper outlines the methodology, including patient selection, CD34+ cell mobilisation, scaffold preparation, injection protocols, clinical assessments, data collection and safety monitoring. The anticipated results, discussion and conclusion will offer insight into the clinical significance and potential impact of ICS-001 as a pioneering angiogenic therapy for CLTI.
Ethics and dissemination: The institutional review boards of all participating hospitals approved this study protocol (latest version V.6.0, 5 June 2025). Final data will be made publicly available. A report detailing the study results will be submitted for publication in an appropriate peer-reviewed journal.
Data availability statement: Data are available on reasonable request. Technical appendix, statistical code is available by contacting the corresponding author. This is an open access article distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) licence, which permits others to distribute, remix, adapt, build on this work non-commercially, and licence their derivative works on different terms, provided the original work is properly cited, appropriate credit is given, any changes made indicated and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/ TRIAL REGISTRATION NUMBER: jRCT2052230115, Japan Registry of Clinical Trials.
Keywords: Cardiovascular Disease; Chronic Limb-Threatening Ischemia; Vascular medicine.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: YK is an employee of Bio X Inc, a manufacturer of an investigational medical device ICS-001. The authors other than YK declare no conflicts of interest related to this study.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical