Targeted degradation of endogenous YAP by nanobody bioPROTAC inhibits tumor progression
- PMID: 41130953
- PMCID: PMC12549912
- DOI: 10.1038/s41467-025-64426-7
Targeted degradation of endogenous YAP by nanobody bioPROTAC inhibits tumor progression
Abstract
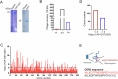
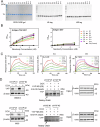
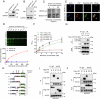
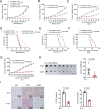
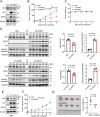
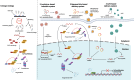
Yes-associated protein (YAP), a key effector of the Hippo pathway, regulates gene expression and promotes tumorigenesis. YAP is conventionally considered "undruggable", however, targeted protein degradation offers a promising approach to address the challenges associated with targeting this oncogenic protein. In this study, through naïve nanobody phage library screening, we identify multiple nanobodies against human YAP with high affinity and specificity. The YAP nanobody is then fused to the RING domain of RNF4, creating a bio-Proteolysis-Targeting Chimera (bioPROTAC) molecule capable of selectively targeting endogenous YAP for ubiquitin-mediated degradation. Notably, the constructed YAP bioPROTAC demonstrates significant YAP degradation and anticancer efficacy in various YAP-dependent cancers both in vitro and in vivo. Nanoparticles and adeno-associated virus (AAV) can effectively deliver the encoding gene of YAP bioPROTAC, achieving YAP degradation in tumors. Collectively, our study provides a proof-of-concept that the YAP nanobody-bioPROTAC approach can effectively degrade endogenous YAP via the ubiquitin-proteasome system, highlighting a feasible strategy for "undruggable" YAP-dependent cancers.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Ma, S., Meng, Z., Chen, R. & Guan, K. L. The Hippo pathway: biology and pathophysiology. Annu. Rev. Biochem.88, 577–604 (2019). - PubMed
-
- Franklin, J. M., Wu, Z. & Guan, K. L. Insights into recent findings and clinical application of YAP and TAZ in cancer. Nat. Rev. Cancer23, 512–525 (2023). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

