FAP+ fibroblasts orchestrate tumor microenvironment remodeling in renal cell carcinoma with tumor thrombus
- PMID: 41130981
- PMCID: PMC12549817
- DOI: 10.1038/s41467-025-64447-2
FAP+ fibroblasts orchestrate tumor microenvironment remodeling in renal cell carcinoma with tumor thrombus
Abstract
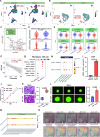
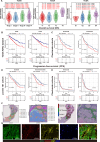
Tumor thrombus (TT) worsens prognosis and complicates surgery in renal cell carcinoma (RCC), yet its formation mechanisms remain unclear. Here, we perform integrative single-cell and spatial transcriptomic analyses on 71 tissues and 48 sections from RCC patients with or without TT. The cellular and spatial atlas reveals distinct TT-associated tumor microenvironment remodeling characterized by the enrichment of FAP+ fibroblasts. These FAP+ fibroblasts are spatially contiguous to aggressive cancer cells and promote their malignant phenotypes in vitro. Their abundance inversely correlates with functional NK cells, suggesting roles in tumor invasion and immune evasion. Furthermore, single-cell multiomics analysis identifies tumor pericytes as a source of FAP+ fibroblasts and delineates transcription factor dynamics underlying pericyte-fibroblast transition. Finally, high levels of FAP+ fibroblasts are associated with poor prognosis and predict a weaker response to anti-VEGF-based therapy. In conclusion, our study highlights FAP+ fibroblasts as drivers of aggressive RCC with TT, suggesting potential therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74, 12–49 (2024). - PubMed
-
- Lardas, M. et al. Systematic review of surgical management of nonmetastatic renal cell carcinoma with vena caval thrombus. Eur. Urol.70, 265–280 (2016). - PubMed
-
- Martinez-Salamanca, J. I. et al. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur. Urol.59, 120–127 (2011). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

