A US case-control study to estimate infant group B streptococcal disease serological thresholds of risk-reduction
- PMID: 41130982
- PMCID: PMC12549997
- DOI: 10.1038/s41467-025-64324-y
A US case-control study to estimate infant group B streptococcal disease serological thresholds of risk-reduction
Abstract
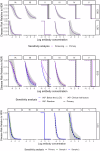
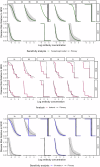
Maternal vaccines to prevent infant Group B Streptococcus (GBS) disease have progressed through phase II development and may be licensed based on immunologic endpoints, which have yet to be approved by regulatory authorities. Here we present a multistate case control study to characterize the relationship between serotype-specific anti-capsular polysaccharide (CPS) immunoglobulin G concentrations near birth and infant GBS disease risk reduction. Antibody concentration distributions are significantly lower for cases (n = 643) than controls (n = 2801) and serologic thresholds varied by serotype and age at onset, with 80% serotype-specific protective thresholds ranging from 0.52 to 2.49 mcg/mL for early-onset disease (EOD; <7 days old) and 0.02 to 0.14 mcg/mL for late-onset disease (LOD; 7-89 days old). Our study provides the most robust data to date that protection thresholds vary by serotype and are notably lower for LOD than EOD, thereby informing potential serological endpoints for phase III trials evaluating CPS-based maternal GBS vaccine candidates.
© 2025. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: Dr. Harrison has served on scientific advisory boards and/or given lectures for Sanofi Pasteur, Pfizer, and GSK, and has served on a data and safety monitoring board for Merck. He receives no compensation other than reimbursement for any travel expenses. The remaining authors declare no competing interests.
Figures
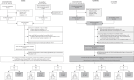
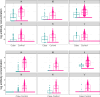
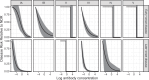

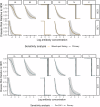
References
-
- World Health Organization. Newborn infections, https://www.who.int/teams/maternal-newborn-child-adolescent-health-and-a... (2025).
-
- Hasperhoven, G., Al-Nasiry, S., Bekker, V., Villamor, E. & Kramer, B. Universal screening versus risk-based protocols for antibiotic prophylaxis during childbirth to prevent early-onset group B streptococcal disease: a systematic review and meta-analysis. BJOG: Int. J. Obstet. Gynaecol.127, 680–691 (2020). - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

