A glial circadian gene expression atlas reveals cell-type and disease-specific reprogramming in response to amyloid pathology or aging
- PMID: 41131361
- PMCID: PMC12586161
- DOI: 10.1038/s41593-025-02067-1
A glial circadian gene expression atlas reveals cell-type and disease-specific reprogramming in response to amyloid pathology or aging
Abstract
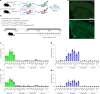
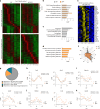
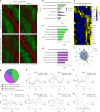
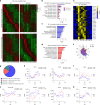
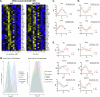
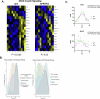
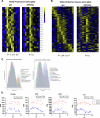
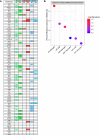
While circadian rhythm disruption may promote neurodegenerative disease, the impact of aging and neurodegenerative pathology on circadian gene expression patterns in different brain cell types remains unknown. Here we used a translating ribosome affinity purification to identify the circadian translatomes of astrocytes, microglia and bulk tissue in healthy mouse cortex and in the settings of amyloid-β plaque pathology or aging. We show that glial circadian translatomes are highly cell-type-specific and exhibit profound, context-dependent reprogramming in response to amyloid pathology or aging. Transcripts involved in glial reactivity, immunometabolism and proteostasis, as well as nearly half of all Alzheimer's disease risk genes, displayed circadian oscillations, many of which were altered by pathology. Microglial oxidative stress and amyloid phagocytosis showed temporal variation in gene expression and function. Thus, circadian rhythms in gene expression are cell-dependent and context dependent, and provide important insights into glial function in health, Alzheimer's disease and aging.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures









Update of
-
A glial circadian gene expression atlas reveals cell type and disease-specific reprogramming in response to amyloid pathology or aging.bioRxiv [Preprint]. 2024 Jun 1:2024.05.28.596297. doi: 10.1101/2024.05.28.596297. bioRxiv. 2024. Update in: Nat Neurosci. 2025 Nov;28(11):2366-2379. doi: 10.1038/s41593-025-02067-1. PMID: 38853870 Free PMC article. Updated. Preprint.
References
-
- Panda, S. et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell109, 307–320 (2002). - PubMed
MeSH terms
Substances
Grants and funding
- R01 AG054517/AG/NIA NIH HHS/United States
- R01 AG068577/AG/NIA NIH HHS/United States
- R01NS102272/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- T32 AG058518/AG/NIA NIH HHS/United States
- R01AG054517/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R01AG054517/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- P30 CA091842/CA/NCI NIH HHS/United States
- R01 NS102272/NS/NINDS NIH HHS/United States
- T32AG058518/U.S. Department of Health & Human Services | NIH | National Institute on Aging (U.S. National Institute on Aging)
- R00AG061231/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- R00 AG061231/AG/NIA NIH HHS/United States
- R01AG068577/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

