Biopsy-proven acute eosinophilic myocarditis as the initial manifestation of severe primary Sjögren's syndrome: a case report
- PMID: 41132250
- PMCID: PMC12541781
- DOI: 10.3389/fcvm.2025.1683444
Biopsy-proven acute eosinophilic myocarditis as the initial manifestation of severe primary Sjögren's syndrome: a case report
Abstract
Background: Primary Sjögren's syndrome (pSS) is a chronic autoimmune inflammatory disorder primarily affecting the exocrine glands. A subset of patients exhibits extraglandular manifestations, including cardiovascular involvement. Among them, myocarditis is a rare complication, and its pathogenesis remains poorly understood.
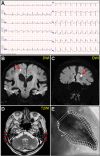
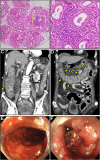
Case presentation: An 85-year-old man with a persistent dry mouth was admitted to our hospital with high-grade fever, nausea, fatigue, and urinary disturbance. On day 2, the patient developed multiple cerebral infarctions and bilateral acute otitis media. Fever and inflammatory response without leukocytosis and cardiac imaging findings indicative of active myocarditis, and normal cardiac function suggested acute viral myocarditis, for which supportive treatments were initiated. On day 6, the patient experienced acute heart failure with severely reduced ejection fraction and cardiogenic shock. An endomyocardial biopsy was performed following transient peripheral eosinophilia in serial blood samples, which revealed acute eosinophilic myocarditis (AEM). A thorough diagnostic evaluation for eosinophilia revealed pSS, leading to the final diagnosis of pSS-associated AEM. Systemic high-dose corticosteroid treatment improved the general condition of the patient, except for a left ventricular apical aneurysm. Nevertheless, the patient's post-treatment hospital course was complicated by serious digestive involvement, leading to death from septic shock.
Conclusions: To our knowledge, this is the first case of severe pSS complicated by AEM. This case highlights the importance of early therapeutic intervention for AEM and early comprehensive surveillance of systemic organs for pSS. Furthermore, this case provides new insights into the pathogenesis of pSS-associated myocarditis.
Keywords: acute eosinophilic myocarditis; acute otitis media; digestive involvement; primary Sjögren's syndrome; ulcerative colitis.
© 2025 Hashimoto, Yamamoto, Isogai, Ikeda, Hamada, Dai and Hashimoto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Shiboski CH, Shiboski SC, Seror R, Criswell LA, Labetoulle M, Lietman TM, et al. 2016 American college of rheumatology/European league against rheumatism classification criteria for primary sjogren’s syndrome: a consensus and data-driven methodology involving three international patient cohorts. Ann Rheum Dis. (2017) 76:9–16. 10.1136/annrheumdis-2016-210571 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

