The effect of tislelizumab on complete and pathological complete response in non-small cell lung cancer: a systematic review and meta-analysis
- PMID: 41132709
- PMCID: PMC12540071
- DOI: 10.3389/fonc.2025.1657282
The effect of tislelizumab on complete and pathological complete response in non-small cell lung cancer: a systematic review and meta-analysis
Abstract
Background: Immune checkpoint inhibitors have transformed non-small cell lung cancer (NSCLC) treatment, and while overall survival (OS) and progression-free survival (PFS) are well-established, a comprehensive meta-analysis focusing on complete response (CR) and pathological complete response (pCR) with tislelizumab-based therapies in NSCLC is lacking.
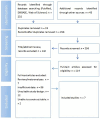
Methods: This systematic review and meta-analysis was conducted following PRISMA guidelines. A thorough literature search was performed across PubMed, Embase, and Web of Science. We included both randomized controlled trials and observational studies of tislelizumab in NSCLC, focusing on extracting data for radiological complete response (CR, based on RECIST 1.1 criteria) and pathological complete response (pCR, defined as absence of residual invasive cancer in resected surgical specimens). Risk of bias was assessed using the Cochrane Collaboration's tool and the Newcastle-Ottawa Scale. Statistical analyses were performed using the 'meta' package in R. 95% confidence intervals (CIs) and odds ratios (ORs) were calculated for CR and pCR, and subgroup analyses were conducted.
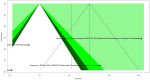
Results: 7 studies were enrolled in the meta-analysis. The results on pCR showed significant heterogeneity (I2 = 92.5%), with a random effects OR of 2.1103 (95% CI: 0.5195 to 8.5727). Subgroup analysis for pCR by disease type revealed a statistically significant difference between NSCLC and SCC only subgroups under the common effect model (p < 0.001). Furthermore, the pCR subgroup analysis by comparator drug showed a statistically significant difference (p < 0.0001) between Pembrolizumab+Chemotherapy (OR 0.6968, 95% CI: 0.3803 to 1.2767) and Chemotherapy alone (OR 7.3123, 95% CI: 2.9204 to 18.3092). For CR, the meta-analysis demonstrated minimal heterogeneity (I2 = 0.0%), yielding a significant random effects OR of 2.6277 (95% CI: 1.2858 to 5.3699). Subgroup analysis for CR comparing tislelizumab plus chemotherapy to chemotherapy alone showed a significant advantage (OR 3.8690, 95% CI: 1.5423 to 9.7059).
Conclusion: Tislelizumab combined with chemotherapy significantly improves CR rates in NSCLC compared to chemotherapy alone. While pCR data exhibit high heterogeneity, the findings highlight tislelizumab's role in achieving deep tumor responses.
Keywords: NSCLC; complete response; meta-analysis; pathological complete response; tislelizumab.
Copyright © 2025 Feng, Yan, Gao, Li, Sarah, Anna and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


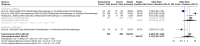




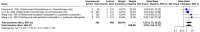
References
-
- Girard N, Han J-Y, Soo RA, Wang K, Tang W, Nikolaidis GF, et al. Comparative effectiveness and safety of tislelizumab versus other anti-PD-(L)1 agents in first- and subsequent lines in locally advanced or metastatic non-small cell lung cancer: Systematic literature review and network meta-analysis. Lung Cancer (Amsterdam Netherlands). (2025) 201:108450. doi: 10.1016/j.lungcan.2025.108450, PMID: - DOI - PubMed
-
- Daei SA, ZareDini M, Fazlollahi A, Sarkesh A, Naseri A, Mousavi SE, et al. The safety and efficacy of tislelizumab, alone or in combination with chemotherapy, for the treatment of non-small cell lung cancer: a systematic review of clinical trials. BMC pulmonary Med. (2023) 23:495. doi: 10.1186/s12890-023-02755-3, PMID: - DOI - PMC - PubMed
-
- Lococo F, Sassorossi C, Nachira D, Chiappetta M, Petracca CL, Vita E, et al. Prognostic factors and long-term survival in locally advanced NSCLC with pathological complete response after surgical resection following neoadjuvant therapy. Cancers. (2020) 12:3572. doi: 10.3390/cancers12123572, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

