Expression of YAP1 delineates distinct subtypes of pulmonary large cell neuroendocrine carcinoma with divergent therapeutic implications
- PMID: 41132957
- PMCID: PMC12541834
- DOI: 10.21037/tlcr-2025-491
Expression of YAP1 delineates distinct subtypes of pulmonary large cell neuroendocrine carcinoma with divergent therapeutic implications
Abstract
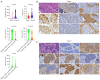
Background: Molecular subtyping [Type I (non-small cell lung carcinoma-like, NSCLC-like) and Type II (small cell lung carcinoma-like, SCLC-like)] and YAP1 expression analysis show promise for precision oncology in pulmonary large cell neuroendocrine carcinoma (LCNEC). However, their clinical utility and prognostic value remain unvalidated, and the aim of this study is to evaluate the disparity of clinicopathologic features, survival outcomes and treatment-relevant biomarkers between these two classifications.
Methods: We retrospectively analyzed 42 surgically resected pulmonary LCNECs. Using next-generation sequencing (NGS) and immunohistochemistry on archival specimens, we classified tumors by molecular subtype and YAP1 protein expression. Clinicopathological features, survival outcomes, and therapeutic biomarkers were compared between these classifications.
Results: Molecular subtyping stratified 61.5% (24/39) as Type I and 38.5% (15/39) as Type II LCNECs. YAP1 immunoreactivity was positive in 71.4% (30/42) of specimens, defining YAP1-negative (n=12) and YAP1-positive (n=30) cohorts. No significant association existed between YAP1 status and molecular subtypes (P=0.15). ASCL1 overexpression (P=0.02) and diffuse prominent nucleoli (P=0.04) were enriched in YAP1-negative tumors, whereas NEUROD1-positive tumors favored YAP1 expression (P=0.04). Type II tumors exhibited elevated POU2F3 expression (vs. Type I, P=0.04) and higher tumor mutation burden (TMB) (P=0.002). YAP1 negativity correlated with upregulated ASCL1 (P=0.003) and DLL3 (P=0.003), whereas positivity associated with major histocompatibility complex class II (MHC II) overexpression (P=0.04). Quantitative analysis revealed that YAP1 expression level correlated positively with NEUROD1 (rho=0.349, P=0.02) but inversely with ASCL1/MHC I (rho=-0.326, P=0.04; rho=-0.338, P=0.03). Neither molecular subtype nor YAP1 status significantly impacted survival.
Conclusions: YAP1 expression was independent of molecular subtypes. YAP1-positive tumors may exhibit enhanced responsiveness to immunotherapy/NEUROD1-targeted therapy, while YAP1-negative tumors could benefit from ASCL1/DLL3-targeted approaches. Prospective validation is warranted.
Keywords: DLL3; Pulmonary large cell neuroendocrine carcinoma (pulmonary LCNEC); biomarkers; immunotherapy; molecular subtype.
Copyright © 2025 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-2025-491/coif). The authors have no conflicts of interest to declare.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials
