Evaluation of Peptide Nucleic Acid Encapsulation in Ferritin Nanocages for Gene Silencing Applications
- PMID: 41134300
- PMCID: PMC12606638
- DOI: 10.1021/acs.biomac.5c01489
Evaluation of Peptide Nucleic Acid Encapsulation in Ferritin Nanocages for Gene Silencing Applications
Abstract
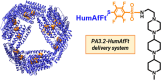
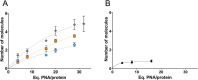
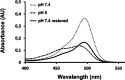
Peptide nucleic acids (PNAs) feature a neutral peptide-like backbone, providing nuclease resistance and potential for precision medicine and diagnostics through specific DNA and RNA binding. However, their therapeutic use is hindered by poor solubility and cell permeability. In this study, we demonstrated that negatively charged PNAs can be readily loaded into the polycationic Humanized Archaeoglobus Ferritin, namely, PA3.2-HumAfFt bioconjugate system, following a divalent-cation-triggered oligomerization technique. The versatility of PNA chemistry enabled the production of synthetic nucleic acid homologues with varying lengths and charges, ranging from positive to negative. We evaluated the loading performance of HumAfFt with and without chemical modifications and investigated the release dynamics of PNAs under conditions simulating the intracellular environment. Our findings demonstrated the effective uptake, release, and biological activity of PNAs in cancer cells, notably silencing the GAPDH gene with good efficiency. This evaluation paves the way for optimizing PNA-based therapeutics and broadening their applications.
Figures


References
-
- Cesaro E., Falanga A. P., Catapano R., Greco F., Romano S., Borbone N., Pastore A., Marzano M., Chiurazzi F., D’Errico S., Piccialli G., Oliviero G., Costanzo P., Grosso M.. Exploring a peptide nucleic acid-based antisense approach for CD5 targeting in chronic lymphocytic leukemia. PLoS One. 2022;17(3):e0266090. doi: 10.1371/journal.pone.0266090. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

