Beta blockers and hypertrophic obstructive cardiomyopathy: a systematic review and meta-analysis
- PMID: 41136226
- PMCID: PMC12557796
- DOI: 10.1136/openhrt-2025-003460
Beta blockers and hypertrophic obstructive cardiomyopathy: a systematic review and meta-analysis
Abstract
Background: Since the 1960s, beta blockers have been used to treat hypertrophic obstructive cardiomyopathy (HOCM), a genetic disorder causing abnormal heart muscle thickening. This systematic review evaluates their efficacy across clinical outcomes.
Methods: Registered on PROSPERO (CRD42022344255), searches were performed in June 2022 and updated in September 2025 across MEDLINE, Embase, CINAHL and PubMed. Two reviewers independently screened studies. Meta-analysis was undertaken when ≥3 comparable datasets were available; otherwise, narrative synthesis was used.
Results: 21 studies including 775 adults met inclusion criteria. Beta blockers significantly reduced left ventricular outflow tract (LVOT) gradient (Standardised mean difference (SMD) -1.57; 95% CI -2.07 to -1.07) and heart rate (SMD -1.19; 95% CI -2.24 to -0.14). Sensitivity analyses confirmed the robustness of the LVOT effect, while heart rate effects remained heterogeneous. Improvements in New York Heart Association class, exercise tolerance and symptom burden were consistently reported, although data were subjective and small in scale. Mortality evidence was limited to two retrospective cohorts with divergent findings.
Conclusions: Beta blockers provide consistent haemodynamic and symptomatic benefits in HOCM, but most evidence derives from small, older studies with high risk of bias and limited survival data. Contemporary, adequately powered randomised controlled trials are required to define optimal agent selection, dosing and long-term outcomes.
Prospero registration number: CRD42022344255.
Keywords: Cardiomyopathy, Hypertrophic; Meta-Analysis; Pharmacology, Clinical; Systematic Reviews as Topic.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures


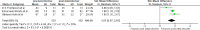
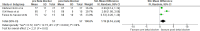
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
