RIPK3 promotes skin inflammation by enhancing IL-36α signaling and necroptosis in keratinocytes
- PMID: 41136377
- PMCID: PMC12552518
- DOI: 10.1038/s41419-025-08096-9
RIPK3 promotes skin inflammation by enhancing IL-36α signaling and necroptosis in keratinocytes
Abstract
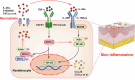
Psoriasis is a chronic inflammatory skin disease characterized by complex pathogenesis involving multiple factors. Keratinocytes, as key structural components, play a critical role in immune regulation and contribute to disease progression through interactions with various immune cells. Receptor-interacting protein kinase 3 (RIPK3) is well-known for its role in necroptosis, acting alongside RIPK1 and mixed-lineage kinase domain-like (MLKL). While studies have shown that inhibitors of necroptosis could alleviate psoriasis-like skin inflammation, direct genetic evidence of RIPK3 is lacking. Furthermore, recent studies have highlighted RIPK3's independent biological functions beyond necroptosis, yet its pathological role in inflammatory skin disease remains poorly understood. This study aimed to elucidate the pathological role of RIPK3 in the progression of skin inflammation, particularly in keratinocytes. We demonstrated that RIPK3 expression was significantly upregulated in psoriasis patients and mice with imiquimod (IMQ)-induced skin inflammation. Importantly, keratinocyte-specific knockout of RIPK3 using gene-editing tools significantly alleviated IMQ-induced skin inflammation in mice. Interestingly, the absence of RIPK3 not only inhibited necroptosis and associated inflammatory responses but also significantly reduced interleukin-36α (IL-36α) expression in keratinocytes. IL-36α, known to drive skin inflammation, promote immune cell recruitment, and disrupt the epidermal barrier, is a critical mediator of inflammatory skin disease pathogenesis. Further investigation using MLKL-knockout mice and keratinocytes revealed that RIPK3 regulates the IL-36α/NF-κB signaling axis through an MLKL-independent mechanism. Collectively, our findings uncover a dual pathogenic role for RIPK3 in skin inflammation: promoting inflammation through both canonical necroptosis and a distinct, non-necroptotic pathway that drives IL-36α activation. These insights not only identify RIPK3 as a potential therapeutic target for psoriasis-like skin inflammation but also uncover its previously unappreciated roles in inflammatory diseases beyond necroptosis.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethical approval: All animal experimental procedures were ethically reviewed and approved by the Institutional Animal Care and Use Committee of the Shanghai Institute of Materia Medica and followed the guidelines set by the Association Assessment and Accreditation of Laboratory Animals Care International.
Figures

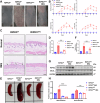
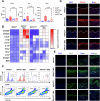

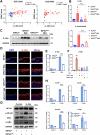
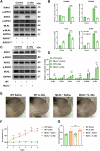
References
-
- Benhadou F, Mintoff D, Del Marmol V. Psoriasis: Keratinocytes or Immune Cells - Which Is the Trigger?. Dermatology. 2019;235:91–100. - PubMed
MeSH terms
Substances
Grants and funding
- No. 82173822/National Natural Science Foundation of China (National Science Foundation of China)
- No. 82173822/National Natural Science Foundation of China (National Science Foundation of China)
- No. 82173822/National Natural Science Foundation of China (National Science Foundation of China)
- No. 82173822/National Natural Science Foundation of China (National Science Foundation of China)
- No. 82173822/National Natural Science Foundation of China (National Science Foundation of China)
- 20JC1418000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20JC1418000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20JC1418000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20JC1418000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
- 20JC1418000/Science and Technology Commission of Shanghai Municipality (Shanghai Municipal Science and Technology Commission)
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

