Development of [18F]ACI-19626 as a first-in-class brain PET tracer for imaging TDP-43 pathology
- PMID: 41136425
- PMCID: PMC12552610
- DOI: 10.1038/s41467-025-64540-6
Development of [18F]ACI-19626 as a first-in-class brain PET tracer for imaging TDP-43 pathology
Abstract
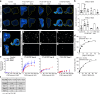
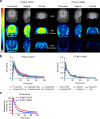
Aggregated TDP-43 is a hallmark of frontotemporal dementia (FTD), amyotrophic lateral sclerosis (ALS), and limbic-predominant age-related TDP-43 encephalopathy (LATE), and a common co-pathology in other neurodegenerative diseases. Currently, no specific biomarkers exist to assess TDP-43 pathology in vivo. We developed two small-molecule radiopharmaceuticals, [18F]ACI-19278 and [18F]ACI-19626, for visualizing TDP-43 inclusions by positron emission tomography (PET). Both ligands bind with high affinity to aggregated, but not soluble, TDP-43 in patient brain samples from diverse TDP-43 proteinopathies, including frontotemporal lobar degeneration with TDP-43 pathology (FTLD-TDP), ALS, and LATE, and in cell models. Both compounds display excellent selectivity for TDP-43 over Aβ, Tau, and α-synuclein aggregates. In non-human primates, [18F]ACI-19278 and [18F]ACI-19626 show a pharmacokinetic profile suitable for brain PET imaging (rapid brain uptake; fast and complete washout). ACI-19278 and ACI-19626 are promising first-in-class TDP-43 PET tracers with the potential to revolutionize the diagnosis and treatment of neurodegenerative proteinopathies, enabling a precision medicine approach.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: EV is a consultant of AC Immune SA. EC, ND, DC, TM, JKB, MR, AMS, TJA, CD, MYR, HK, FCA, RLC, TA, AP, MKV and TS are or were employees of AC Immune SA at the time of their contribution to this manuscript. The remaining authors declare no competing interests.
Figures


References
-
- Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science314, 130–133 (2006). - PubMed
-
- Arai, T. et al. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys. Res. Commun.351, 602–611 (2006). - PubMed
-
- Arai, T. et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol.117, 125–136 (2009). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

