Implementing integrated genomic risk assessments for breast cancer: lessons learned from the Electronic Medical Records and Genomics study
- PMID: 41140463
- PMCID: PMC12552095
- DOI: 10.1093/jamiaopen/ooaf113
Implementing integrated genomic risk assessments for breast cancer: lessons learned from the Electronic Medical Records and Genomics study
Abstract
Objectives: To implementation an automated multi-institutional pipeline that delivers breast-cancer risk integrated with polygenic risk scores, monogenic variants, family history, and clinical factors, emphasizing operational challenges and their solutions.
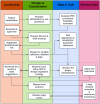
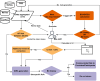
Materials and methods: A five-stage process was executed at ten sites. Data streams from REDCap surveys, PRS and monogenic reports, and MeTree pedigrees were normalized and forwarded through a REDCap plug-in to the CanRisk API.
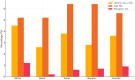
Results: Integrated risk was returned to >10 000 women; 3.6% were ≥25 % lifetime risk and 0.9% carried pathogenic variants. Pipeline generated score aligns well with manual generated ones. Major barriers such as heterogeneous pedigree formats, missing data, edge-case handling, and evolving model versions were identified and resolved through mapping rules, imputations, and iterative testing.
Discussion: Cross-platform data harmonization and stakeholder alignment were decisive for success. Borderline-risk communication and model-version drift remain open issues.
Conclusion: Large-scale PRS-integrated breast-cancer risk reporting is feasible but requires robust interoperability standards and iterative governance.
Keywords: breast cancer; clinical workflow automation; integrated risk score; polygenic risk score.
© The Author(s) 2025. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Conflict of interest statement
A.C.A. and T.C. are listed as creators of BOADICEA, which has been licensed by Cambridge Enterprise (University of Cambridge); N.S.A.-H. is an employee of the 23andMe Research Institute; E.E.K. has received personal fees from Regeneron Pharmaceuticals, 23&Me, Allelica, and Illumina; E.E.K. has received research funding from Allelica; and serves on the advisory boards for Encompass Biosciences, Overtone, and Galateo Bio.
Figures
Update of
-
Implementing Integrated Genomic Risk Assessments for Breast Cancer: Lessons Learned from the eMERGE Study.medRxiv [Preprint]. 2025 May 23:2025.05.22.25328180. doi: 10.1101/2025.05.22.25328180. medRxiv. 2025. Update in: JAMIA Open. 2025 Oct 23;8(5):ooaf113. doi: 10.1093/jamiaopen/ooaf113. PMID: 40661297 Free PMC article. Updated. Preprint.
References
LinkOut - more resources
Full Text Sources
