Anti-CD38 Daratumumab Treatment of Chronic Active Antibody-Mediated Kidney Allograft Rejection
- PMID: 41141546
- PMCID: PMC12546618
- DOI: 10.1016/j.ekir.2025.07.009
Anti-CD38 Daratumumab Treatment of Chronic Active Antibody-Mediated Kidney Allograft Rejection
Abstract
Introduction: Chronic active (c) antibody-mediated rejection (ABMR) (cABMR) remains the leading cause of graft loss in kidney transplant recipients (KTRs). The efficacy of subcutaneous daratumumab, an anti-CD38 monoclonal antibody targeting plasma cells and natural killer (NK) cells, to treat cABMR was studied.
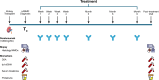
Methods: A retrospective chart review was performed on KTRs with cABMR diagnosed > 6 months after transplantation and treated with a flat dose of subcutaneous daratumumab 1800 mg weekly for 4 weeks followed by 3 quarterly doses. The patients were monitored before and after treatment using estimated glomerular filtration rate (eGFR), urine albumin-to-creatinine ratio (UACR), renal-biopsy, donor-derived cell-free DNA (dd-cfDNA) and donor-specific antibody (DSA) levels. Adverse events were monitored.
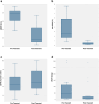
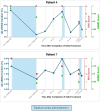
Results: Sixteen KTR with cABMR (median time from transplantation to treatment: 9 years) were treated with daratumumab. No major adverse events occurred. After 10 months, biopsy histology showed improvement in microvascular inflammation scores in 13 of 16 patients and molecular ABMR scores decreased (median 74% decline), with 8 of 16 patients experiencing complete molecular remission of ABMR. eGFR levels remained stable, UACR improved in 11 of 16 patients and dd-cfDNA significantly decreased (median decrease: 85%). DSA became negative in 2 of 12 previously positive patients. After completion of treatment, patients were monitored quarterly with eGFR, UACR, and dd-cfDNA. Two patients showed recurrence of cABMR based on dd-cfDNA, were retreated, and showed stabilization/improvement.
Conclusion: Subcutaneous daratumumab may be an effective treatment for cABMR; larger randomized trials are warranted to study its role in the treatment for cABMR in KTRs. dd-cfDNA may be a useful monitoring tool to predict and detect relapses.
Keywords: anti-CD38 monoclonal antibodies; chronic active antibody-mediated rejection; kidney transplantation; retrospective chart review.
© 2025 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

