A 3D spheroid model for assessing nanocarrier-based drug delivery to solid tumors
- PMID: 41142464
- PMCID: PMC12552133
- DOI: 10.1038/s44385-025-00041-x
A 3D spheroid model for assessing nanocarrier-based drug delivery to solid tumors
Abstract
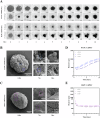
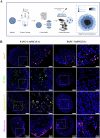
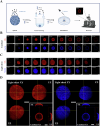
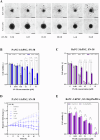
3D spheroid culture has emerged as a valuable tool for studying complex intratumoral processes and screening novel therapeutics in vitro. However, spheroids face reproducibility and data interpretation issues, which limit their utility. This work describes a simple and reproducible co-culture spheroid model compatible with high-throughput screening designed to study pancreatic ductal adenocarcinoma (PDAC), a highly therapy-resistant cancer. These spheroids, composed of both cancer and stromal cells, recapitulate key features of PDAC which are difficult to study in traditional 2D cell culture, including hypoxia, fibrosis and chemoresistance. Light sheet microscopy is used to study the tissue penetration of polymeric Pluronic® F127-polydopamine (PluPDA) nanocarriers (NCs) in this model while showing that confocal microscopy is not suitable for such studies and should be avoided. Additionally, the efficacy of PluPDA NCs loaded with the chemotherapeutic SN-38 is demonstrated in 3D, justifying their advancement to in vivo trials. Finally, the methodology is extended to generate lung adenocarcinoma spheroids, showcasing the versatility of this approach. Overall, this research is intended to serve as a robust platform for studying NCs under physiologically relevant conditions, ultimately resulting in a more efficient clinical translation pathway for nanomaterials.
Keywords: Cancer models; Nanoparticles; Pancreatic cancer.
© The Author(s) 2025.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures



References
-
- Dowden, H. & Munro, J. Trends in clinical success rates and therapeutic focus. Nat. Rev. Drug Discov.18, 495–496 (2019). - PubMed
-
- Elmore, L. W. et al. Blueprint for cancer research: critical gaps and opportunities. CA Cancer J. Clin.71, 107–139 (2021). - PubMed
-
- Yan, H. H. N., Chan, A. S., Lai, F. P.-L. & Leung, S. Y. Organoid cultures for cancer modeling. Cell Stem Cell30, 917–937 (2023). - PubMed
LinkOut - more resources
Full Text Sources
