Management of cancer treatment-related fatigue in advanced breast cancer patients: an expert committee's opinion
- PMID: 41142595
- PMCID: PMC12545012
- DOI: 10.3389/fonc.2025.1617600
Management of cancer treatment-related fatigue in advanced breast cancer patients: an expert committee's opinion
Abstract
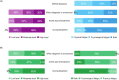
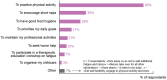
Cancer-related fatigue (CRF) is a frequent and complex adverse event associated with advanced breast cancer (ABC). CRF intensity and impact on a patient's daily life are often exacerbated by cancer treatments. This specific manifestation, known as cancer treatment related fatigue (CTRF), begins with treatment onset and can persist beyond its course. CTRF is not unavoidable; its effect can be reduced through careful management and differential diagnosis from CRF. This article aims to review current recommendations for assessing and managing fatigue and present an expert opinion on priority actionable actions for evaluating and alleviating fatigue in ABC patients. In addition, to better understand the current standard of care and management options for ABC patients with fatigue, a quantitative survey was conducted from July to September 2023 through online standardized questionnaire containing identical questions between oncologists (N = 43) and ABC patients (N = 132) in France. Results confirm fatigue's complexity and multidimensional nature. Insufficient time and lack of communication during consultations contribute to ineffective diagnosis and management of fatigue, highlighting the need for improvement through better communication.
Keywords: adapted physical activity; advanced breast cancer; cancer treatment-related fatigue; cancer-related fatigue; metastatic breast cancer; patient education; physical activity.
Copyright © 2025 Bécourt, Abadie-Lacourtoisie, Calvat, Coudurier-Curveur, Fiteni, Mery, Meynard, Scotté, Vaflard and Zelek.
Conflict of interest statement
SB has received personal fees and non-financial support from Pfizer, Lilly, Novartis, Gilead, Astrazeneca, Daiichi Sankyo. LZ has received personal fees and non-financial support from Gilead, and personal fees from AstraZeneca, Daiichi-Sankyo, Novartis, Lilly and Pfizer. FF has received consulting fees from Astrazeneca, BMS, Clovis pharma, Daiishi-Serono, GSK, Jansen, Lilly, Novartis, Pfizer, Seagen, Gilead, Menarini-Steamline, MSD, Gilead, and support for attending meetings and/or travel from Pfizer, Lilly, Gilead, Astrazeneca. GM has received personal fees from Pfizer, Lilly, Daiichi, Novartis, Eisai, Astrazeneca, and non-financial support from Pfizer, GSK, Gilead. FS has received consulting fees and personal fees from Sanofi, Roche, MSD, Gilead, Norgine, Helsinn, Prostrakan, Leo Pharma, Janssen, Viatris, Pharmanovia, AMGEN, Pierre Fabre Oncologie, Vifor Pharma, Immedica, La Roche Posay, Pfizer, BMS, Daiichi Sankyo, Fresenius Kabi. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Network. NCC . Clinical practice guidelines in oncology (NCCN guidelines®). In: Cancer-related fatigue. Version 2.2025 (2025). Available online at: https://www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf (Accessed September 5, 2025).
Publication types
LinkOut - more resources
Full Text Sources

