Multimodal reprogramming of the tumor microenvironment by MMR and dual checkpoint blockade in hepatocellular carcinoma models
- PMID: 41142777
- PMCID: PMC12546120
- DOI: 10.3389/fimmu.2025.1679665
Multimodal reprogramming of the tumor microenvironment by MMR and dual checkpoint blockade in hepatocellular carcinoma models
Abstract
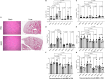
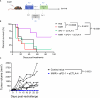
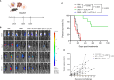
Hepatocellular carcinoma (HCC) is a leading cause of cancer-related death worldwide, thus, there is an urgent need to develop more effective therapeutic options for this dismal condition. Tumor-infiltrating lymphocytes (TILs) are associated with improved response to immune checkpoint blockade in HCC, but their low abundance in most cases limits their therapeutic efficacy. Here, we demonstrate, in mice, that low-dose intratumoral immunovirotherapy with the trivalent measles, mumps, and rubella vaccine (MMR) induces superior tumor-growth delay and extended host survival compared to individually administered vaccines for measles, mumps, or rubella viruses. Further, our results show that MMR therapy synergizes with PD-1 and CTLA-4 blockade to reprogram the tumor microenvironment, resulting in increased CD8+ TIL infiltration and reduced PD-1 expression on TILs, among other effects. These changes in the immunological landscape translated into greater survival and more durable tumor-specific and memory immune responses for hosts. Comprehensive toxicology analysis revealed no evidence of MMR-induced liver or kidney toxicity after intrahepatic administration. This work reinforces an unrecognized role of MMR plus ICB in reprogramming the immune landscape in HCC through multimodal immune activation, providing a strong rationale for further development of MMR-based therapies for HCC.
Keywords: MMR vaccine; hepatocellular carcinoma; immune checkpoint blockade; innate and adaptive immunity modulation; tumor microenvironment.
Copyright © 2025 Tesfay, Cios, Ferdous, Shelton, Mustafa, Simoes, Gokden, Miousse, Krager, Boerma, Urbaniak, Kunthur, Obulareddy, Eichhorn, Post, Chamcheu, Moaven, Chabu, Duda, Conti, Nardo, Govindarajan, Fernandez-Zapico, Roberts, Borad, Cannon, Basnakian and Nagalo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- Asafo-Agyei KO, Samant H. Hepatocellular carcinoma. In: StatPearls. Treasure Island (FL): StatPearls Publishing; (2025)., PMID: - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

