Dihydroorotate dehydrogenase inhibition activates STING pathway and pyroptosis to enhance NK cell-dependent tumor immunotherapy
- PMID: 41144149
- PMCID: PMC12559559
- DOI: 10.1186/s43556-025-00339-7
Dihydroorotate dehydrogenase inhibition activates STING pathway and pyroptosis to enhance NK cell-dependent tumor immunotherapy
Abstract
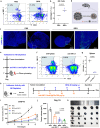
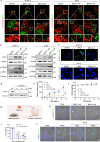
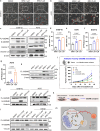
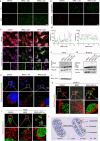
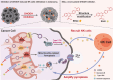
Cancer cells rely heavily on de novo pyrimidine synthesis. Inhibiting pyrimidine metabolism directly suppresses tumor growth and fosters immune activation within the tumor microenvironment. Dihydroorotate dehydrogenase (DHODH) is a key enzyme in the de novo pyrimidine synthesis pathway. Inhibiting DHODH can reverse immune suppression and trigger a mild innate immune response. However, the impact of DHODH inhibition on natural killer (NK) cells remains to be explored. In this study, we found that DHODH inhibition promoted NK cell infiltration into tumors efficiently. Mechanistically, DHODH suppression induced mitochondrial oxidative stress, leading to mitochondrial DNA (mtDNA) release into the cytoplasm through voltage-dependent anion channel (VDAC) oligomerization and caspase-3 activation. This subsequently activated the stimulator of interferon gene (STING) pathway, triggered ferroptosis, and induced gasdermin E (GSDME) mediated pyroptosis in cancer cells. These changes collectively facilitated NK cell recruitment. Furthermore, infiltrated NK cells enhanced GSDME-dependent pyroptosis in tumor cells through granzyme release, establishing a positive feedback loop that amplified anti-tumor immunity. Additionally, we developed EA6, a novel DHODH inhibitor that is more effective at promoting NK cell infiltration. In summary, this study reveals that targeting pyrimidine metabolism activates a novel mechanism involving pyroptosis-ferroptosis crosstalk and STING pathway activation to enhance NK cell-mediated immunity. These finding opens new avenues for enhancing the efficacy of targeted nucleotide metabolism in cancer therapy.
Keywords: CGAS-STING pathway; DHODH; NK cells; Pyrimidine metabolism; Pyroptosis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethics approval was granted by the Ethics Committee of Northwestern Polytechnical University. Animal experiments were carried out according to the Animal Experiment Center of Northwestern Polytechnical University Animal Care and Use Guidelines (NO. 202201173). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Medical
Research Materials
