Association of Increased Central Arterial Stiffness With BBB Disruption in Patients With Reversible Cerebral Vasoconstriction Syndrome
- PMID: 41144838
- PMCID: PMC12570067
- DOI: 10.1212/WNL.0000000000214318
Association of Increased Central Arterial Stiffness With BBB Disruption in Patients With Reversible Cerebral Vasoconstriction Syndrome
Abstract
Background and objectives: Blood-brain barrier (BBB) disruption was found to be critical in the pathogenesis of reversible cerebral vasoconstriction syndrome (RCVS). We hypothesized that increased central arterial stiffness, resulting in the excessive transmission of central pulsatile flow to the dysregulated cerebral microcirculation, may contribute to the disruption of BBB.
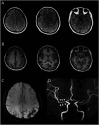
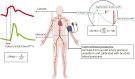
Methods: This is a cross-sectional study. Participants with RCVS in the acute phase were recruited from both outpatient and inpatient settings between January 2018 and September 2020. The diagnosis was established based on clinical presentations and neuroradiologic evidence. Age-matched and sex-matched healthy controls (HCs) were recruited from nearby communities. Patients with RCVS were recruited and underwent 3-dimensional isotropic contrast-enhanced T2 fluid-attenuated inversion recovery imaging to visualize BBB disruption. The central arterial stiffness was assessed with hemodynamic parameters such as carotid-femoral pulse wave velocity (cfPWV), carotid augmentation index (cAI), and central blood pressure (ceBP). Arterial stiffness was compared between patients with RCVS and HCs, and between patients with RCVS with and without BBB disruption.
Results: Sixty-five patients with RCVS (mean age 44.3 ± 9.6 years; 42 women) and 65 HCs (mean age 45.4 ± 8.9 years; 42 women) completed the study. Among the patients with RCVS, 33 exhibited imaging-proven BBB disruption. Those with BBB disruption were older (48.3 ± 9.2 vs 40.2 ± 8.3 years, p < 0.001) and had a higher proportion of women (81.8% vs 46.9%, p = 0.003). Arterial stiffness was increased in patients with RCVS, as indicated by a higher cfPWV compared with controls (10.8 ± 2.5 m/s vs 9.2 ± 2.3 m/s, p < 0.001). Among patients with RCVS, those with BBB disruption had greater central arterial stiffness, reflected by higher cfPWV and cAI (11.4 ± 2.7 m/s vs 10.1 ± 2.1 m/s, p = 0.036; 15 ± 18% vs 2 ± 19%, p = 0.007). There were no significant differences in ceBP between patients with RCVS (regardless of BBB disruption) and HCs.
Discussion: Our study confirmed increased central arterial stiffness in patients with RCVS, especially among those with BBB disruption. These findings suggest a potential contribution of central arterial stiffness and excessive pulsatile flow to cerebral microvascular dysfunction in the pathogenesis of RCVS.
Conflict of interest statement
Y.-H. Ling received funding from Taipei Veterans General Hospital (V114A-002) and Vivian W. Yen Neurological Foundation. S.-J. Wang received funding from the Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan; the National Science and Technology Council, Taiwan (NSTC 110-2321-B-010-005, 111-2314-B-A49-090-MY3, 112-2321-B-075-007, 113-2321-B-A49-017, and 113-2811-B-A49A-012); and Taipei Veterans General Hospital (V113C-120 and V113E004-1). S.-P. Chen received funding from the Brain Research Center, National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan and the National Science and Technology Council, Taiwan (NSTC 110-2326-B-A49A-501-MY3 and 112-2314-B-A49-037-MY3), and Taipei Veterans General Hospital (V112D67-001-MY3-2 & V113C-058). All other authors report no disclosures relevant to the manuscript. Go to
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources
