Immediate Postoperative AbnobaVISCUM® F as an Adjuvant Treatment in Patients Receiving Standard Care after Pancreatic Cancer Resection
- PMID: 41145311
- PMCID: PMC12568445
- DOI: 10.3349/ymj.2024.0493
Immediate Postoperative AbnobaVISCUM® F as an Adjuvant Treatment in Patients Receiving Standard Care after Pancreatic Cancer Resection
Abstract
Purpose: AbnobaVISCUM® F is an anti-malignant tumor agent derived from Viscum album, a mistletoe species parasitic to ash trees. It is known to exert anticancer effects by activating the patient's immune system without directly inducing tumor toxicity. We retrospectively investigated the anticancer effect of AbnobaVISCUM® F in patients with resected pancreatic cancer.
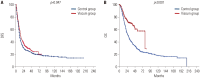
Materials and methods: We reviewed a total of 985 patients who underwent radical resection for pancreatic cancer between January 2005 and August 2022 at Severance Hospital, Seoul, Korea. Patients were divided into two groups based on whether Abnoba VISCUM® F was administered (Viscum group) or not (Control group), and clinicopathologic characteristics, disease-free survival (DFS) and overall survival (OS) were compared after propensity score matching (PSM).
Results: Of the 985 patients, 310 received Viscum therapy at least 12 times (Viscum group), while 590 did not receive it at all (Control group). After PSM, both groups showed similar DFS (p=0.518), whereas the Viscum group showed superior OS (p<0.001). Subgroup analyses revealed better OS for the Viscum group in T1 (p=0.014), T2 (p=0.012), N0 (p=0.010), N1 (p=0.001), R0 resection patients (p<0.001), and in patients who underwent no adjuvant chemotherapy or chemotherapy regimens other than FOLFIRINOX (p<0.001). Multivariable analysis identified Viscum therapy as an independent factor for improved OS (hazard ratio 0.601, p< 0.001), although it did not significantly impact DFS.
Conclusion: Viscum treatment significantly improved OS in patients with completely resected stage I and II pancreatic cancer, particularly among those unable to tolerate adjuvant chemotherapy. This anticancer effect, based on immune enhancement, should be further investigated, and large-scale randomized controlled study is warranted.
Keywords: Viscum album; adjuvant treatment; pancreatic cancer.
© Copyright: Yonsei University College of Medicine 2025.
Conflict of interest statement
The authors have no potential conflicts of interest to disclose.
Figures


References
-
- Ducreux M, Cuhna AS, Caramella C, Hollebecque A, Burtin P, Goéré D, et al. Cancer of the pancreas: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26(Suppl 5):v56–v68. - PubMed
-
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395:2008–2020. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, Asbun H, Bain A, Behrman SW, et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:1028–1061. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

