Rapid nanocatalytic approach for azo dye degradation using bi-ligand nickle based-metal organic frameworks
- PMID: 41146256
- PMCID: PMC12560516
- DOI: 10.1186/s13065-025-01650-8
Rapid nanocatalytic approach for azo dye degradation using bi-ligand nickle based-metal organic frameworks
Abstract
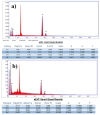
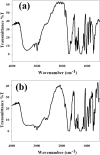
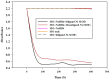
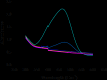
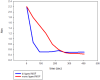
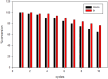
A novel bi-ligand nickel-based metal-organic framework (Ni-BTC-PYDC MOF) was synthesized using benzene tricarboxylic acid (BTC) and pyridine-2,3-dicarboxylic acid (PYDC) as ligands. This MOF showed improved surface area, structural stability, and electron transfer compared to mono-ligand Ni-MOFs. Characterization by SEM, EDX, EDS mapping, XRD, and FT-IR confirmed its enhanced morphology and nickel content. The catalyst rapidly reduced methyl orange (MO) dye in water, achieving rapid and significant decolorization within 90 s using sodium borohydride (NaBH₄) under mild conditions. It maintained high activity over ten reuse cycles with minimal loss, performing best at pH 5 due to efficient hydride generation and proton-assisted electron transfer. These findings demonstrate that the bi-ligand Ni-MOF is a promising, stable, and reusable catalyst for removing toxic azo dyes from wastewater.
Keywords: Azo-dyes; Bi-ligand Ni-MOFs; Environmental remediation; Metal organic frameworks; Methyl orange.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures


References
-
- Mani S, Bharagava R. Textile industry wastewater. Environmental and Health Hazards and Treatment Approaches; 2018.
-
- Khan A, Ju P, Han Z, Ni C. A comprehensive review on adsorptive removal of Azo dyes using functional materials. AQUA - Water Infrastructure Ecosyst Soc. 2024;73:266–85.
-
- Asranudin AS, Purnomo, Holilah D, Prasetyoko N, El Messaoudi AA, Rohmah AR, Putra Hidayat, Subagyo R. Adsorption and biodegradation of the Azo dye Methyl orange using ralstonia Pickettii immobilized in Polyvinyl alcohol (PVA)–alginate–hectorite beads (BHec-RP). RSC Adv. 2024;14:18277–90. - PMC - PubMed
-
- Ali NS, Khader EH, khudhur RH, Abdulrahman MA, Salih IK, Albayati TM. Removal of anionic Azo dye from wastewater using Fe3O4 magnetic nanoparticles adsorbents in a batch system. Desalination Water Treat. 2024;317:100033.
LinkOut - more resources
Full Text Sources
