Alzheimer's Disease Clinical Trial Decision-Making Among Patients with Mild Cognitive Impairment and Their Study Partners
- PMID: 41147833
- PMCID: PMC12593232
- DOI: 10.1080/23294515.2025.2576830
Alzheimer's Disease Clinical Trial Decision-Making Among Patients with Mild Cognitive Impairment and Their Study Partners
Abstract
Background. Prodromal Alzheimer's disease (AD) clinical trials enroll patients with mild cognitive impairment (MCI) and their study partners. This study examined trial enrollment decision-making and risk for misunderstanding trial information presented in an informed consent process for prodromal AD trials.
Methods. We performed structured interviews with patients with MCI and individuals who could serve as their study partners. We presented details of a hypothetical prodromal AD trial, followed by questionnaires to assess involvement of the study partner in trial decisions, patient capacity to consent, and patient health numeracy skills.
Results. Among 65 patient participants, most were male (66%) and non-Hispanic White (88%) with a mean (standard deviation) age of 74.9 (8.4) years. Among 57 study partners, most were spouses (83%), female (74%), and non-Hispanic White (91%) with a mean (standard deviation) age of 70.3 (13.6). Most patient participants (66%) and study partners (72%) reported they would make enrollment decisions in partnership; 28% of patient participants said they would make the decision themselves, with input from a study partner. Twenty-five patient participants (38%) lacked capacity to consent to the trial; 38% demonstrated impaired health numeracy. Seventeen patient participants (26%) demonstrated both a lack of capacity and impaired health numeracy, among whom seven reported they would make enrollment decisions on their own, with input from a study partner.
Conclusions. Some patients with MCI demonstrated a lack of capacity to consent, impaired health numeracy, or both, putting them at risk for misunderstanding trial information in the consent process for a prodromal AD clinical trial.
Keywords: Alzheimer’s disease; clinical trials; decision-making; mild cognitive impairment.
Conflict of interest statement
Disclosure statement
On behalf of all authors, the corresponding author (CGC) states that there is no conflict of interest. Sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; in the preparation of the manuscript; or in the review or approval of the manuscript.
Figures


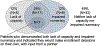
References
-
- Delazer M, Kemmler G, Benke T. 2013. Health numeracy and cognitive decline in advanced age. Aging Neuropsychol Cognit. 20(6):639–659. https://www.tandfonline.com/doi/abs/10.1080/13825585.2012.750261. 10.1080/13825585.2012.750261 - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
