Development and Evaluation of Nystatin-Loaded Novasomal Gel for the Treatment of Candida albicans Infection: In Vitro Microbiological and Skin Compatibility Study
- PMID: 41149379
- PMCID: PMC12564917
- DOI: 10.3390/gels11100774
Development and Evaluation of Nystatin-Loaded Novasomal Gel for the Treatment of Candida albicans Infection: In Vitro Microbiological and Skin Compatibility Study
Abstract
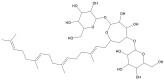
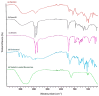
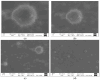
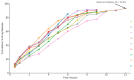
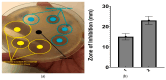
Candida infections pose a significant health threat, and conventional antifungal drugs like nystatin are limited due to poor solubility, skin permeability, and frequent dosage requirements. Nystatin effectively targets Candida species by disrupting cell membranes, but formulation issues hinder clinical use. Lipid-based vesicular carriers, or novasomes, provide controlled, prolonged drug release and enhanced skin penetration. This study focuses on developing nystatin-loaded novasomal gels as an advanced drug delivery system to enhance therapeutic efficacy, bioavailability, and patient compliance. The formulation was prepared using a modified ethanol injection technique, combining stearic acid, oleic acid, Span 60, cholesterol, and Carbopol to produce a stable transdermal gel. Comprehensive in vitro characterization using FTIR, SEM, XRD, and thermal analysis confirmed the chemical compatibility, morphological uniformity, and physical stability of the nystatin-loaded novasomal gel. Entrapment efficiency differed significantly among the formulations (p < 0.05), with F7 achieving the highest value (80%). All formulations maintained pH levels within the skin-friendly range of 5.5 to 7.0. Viscosity measurements, ranging from 3900 ± 110 to 4510 ± 105 cP, confirmed their appropriate consistency for dermal use. Rheological analysis showed a dominant elastic response, as indicated by storage modulus values consistently higher than the loss modulus. Particle size ranged from 4143 to 9570 nm, while PDI values remained below 0.3, reflecting uniform particle distribution. Zeta potential values were strongly negative, supporting physical stability. XRD studies indicated reduced crystallinity of nystatin within the formulations, while FTIR confirmed drug-excipient compatibility. SEM images showed spherical particles within the micrometer range. In vitro release studies demonstrated sustained drug release over 12 h, with F6 releasing the highest amount. The novasomal gel formulations-maintained stability for 30 days, with no notable alterations in pH, viscosity, or entrapment efficiency. Antifungal evaluation showed a larger inhibition zone (23 ± 2 mm) compared with the plain drug solution (15 ± 1.6 mm), while the MIC value was reduced (4.57 µg/mL), indicating greater potency. Skin irritation assessment in rats revealed only minor, temporary erythema, and the calculated Primary Irritation Index (0.22) confirmed a non-irritant profile. These findings suggest that the developed novasomal gel offers a promising approach for enhancing the treatment of fungal infections by enabling prolonged drug release, minimizing dosing frequency, and improving patient compliance.
Keywords: ethanol injection; fungal infections; nystatin; skin compatibility; transdermal drug delivery; vesicular system.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Srikanth K., Gupta V.R.M., Devanna N. Formulation and Evaluation of Nystatin Loaded Niosomes. Int. J. Pharm. Sci. Res. 2013;4:2015.
-
- Yamasaki M., Tamura N., Nakamura K., Sasaki N., Murakami M., Rajapakshage W., Kumara B., Tamura Y., Lim S.Y., Ohta H., et al. Effects and Mechanisms of Action of Polyene Macrolide Antibiotic Nystatin on Babesia gibsoni In Vitro. J. Parasitol. 2011;97:1190–1192. doi: 10.1645/GE-2799.1. - DOI - PubMed
-
- Dos Santos A.G., Marquês J.T., Carreira A.C., Castro I.R., Viana A.S., Mingeot-Leclercq M.P., de Almeida R.F.M., Silva L.C. The Molecular Mechanism of Nystatin Action Is Dependent on the Membrane Biophysical Properties and Lipid Composition. Phys. Chem. Chem. Phys. 2017;19:30078–30088. doi: 10.1039/C7CP05353C. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

