Molecular Profiling of SYT-SSX Fusion Transcripts for Enhanced Diagnosis of Synovial Sarcomas
- PMID: 41149817
- PMCID: PMC12565586
- DOI: 10.3390/jpm15100455
Molecular Profiling of SYT-SSX Fusion Transcripts for Enhanced Diagnosis of Synovial Sarcomas
Abstract
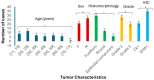
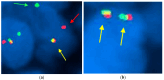
Background/Objectives: Synovial sarcoma (SS) is an aggressive soft-tissue tumor characterized by the chromosomal translocation t(X;18) (p11.2;q11.2), most commonly involving the fusion of the SYT gene on chromosome 18 with the SSX1 or SSX2 genes on chromosome X. This study aims to explore the clinicopathological and molecular characteristics of synovial sarcoma in a cohort of Moroccan patients. Methods: We analyzed 48 cases of synovial sarcoma using formalin-fixed, paraffin-embedded (FFPE) tissue samples. Histological grading was performed according to the FNCLCC system. Immunohistochemical staining was employed to detect cytokeratin (CK) and epithelial membrane antigen (EMA). Molecular analysis included fluorescence in situ hybridization (FISH) to identify SS18 gene rearrangements and reverse transcription-polymerase chain reaction (RT-PCR) to detect SYT-SSX fusion transcripts. Results: Among the cohort, 56% of cases showed SS18 gene rearrangements via FISH, while RT-PCR confirmed the presence of SS18-SSX1 and SS18-SSX2 transcripts in 60% and 32% of cases, respectively. The remainder was classified as undifferentiated sarcoma. Notably, no significant associations were observed between SYT-SSX fusion type and clinicopathological features. Conclusions: These findings underscore the importance of integrating molecular techniques for precise diagnosis in synovial sarcoma. The results align with global patterns, emphasizing the necessity for molecular testing to enhance diagnostic accuracy and informing potential therapeutic advancements.
Keywords: SYT-SSX fusion transcripts; molecular diagnostics; synovial sarcoma.
Conflict of interest statement
Authors declare no conflicts of interest.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

