Efficacy of a Novel PCV2d and Mycoplasma hyopneumoniae Combined Vaccine in Piglets with High and Low Levels of PCV2 Maternally Derived Antibodies at Vaccination
- PMID: 41150462
- PMCID: PMC12568175
- DOI: 10.3390/vaccines13101076
Efficacy of a Novel PCV2d and Mycoplasma hyopneumoniae Combined Vaccine in Piglets with High and Low Levels of PCV2 Maternally Derived Antibodies at Vaccination
Abstract
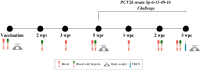
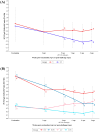
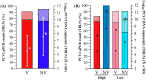
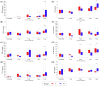
Background/Objectives: Maternally derived antibody (MDA) levels of porcine circovirus 2 (PCV2) may eventually interfere with humoral response and vaccination efficacy. This study aimed to evaluate the efficacy of a ready-to-use PCV2d and Mycoplasma hyopneumoniae combined vaccine in piglets with different PCV2 MDA levels at vaccination in an experimental inoculation with a heterologous viral genotype. Methods: Forty-eight piglets were allocated into vaccinated (V) and non-vaccinated (NV) groups with high (H) and low (L) PCV2 MDA subgroups (H-V, H-NV, L-V, L-NV). At 3 weeks of age, the piglets received either one dose of vaccine or placebo. Five weeks later, all animals were intranasally challenged with a PCV2b inoculum. Body weight was registered at different time points. Blood samples, peripheral blood mononuclear cells and tracheobronchial lymph nodes (TBLN) were collected and used to assess viraemia, viral load, humoral and cellular responses and histological lesions. Results: The V group showed higher PCV2 antibody levels from challenge onwards, along with a lower percentage of viraemic pigs and reduced viral load in serum at 2 and 3 weeks post-challenge (wpc) and in TBLN tissues compared to the NV group. The H-V group had the highest antibody levels post-challenge, showed no detectable viraemia and had a lower overall amount of virus in tissues. The NV group (especially H-NV) exhibited increased levels of IFN-γ, IFN-α and TNF-α post-challenge. Conclusions: The tested vaccine elicited humoral and cellular immune responses and reduced viral presence in serum and tissues, demonstrating efficacy in a PCV2 subclinical infection model despite high MDA levels at the time of vaccination. Understanding both humoral and cellular immune responses according to different MDA levels can help design more effective vaccination strategies against PCV2.
Keywords: cytokines; in situ hybridisation; maternally derived antibodies; porcine circovirus 2; quantitative PCR; vaccine.
Conflict of interest statement
Mònica Sagrera, Laura Garza-Moreno and David Espigares are employees of Ceva Salud Animal.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources

