Oxysterol-binding Protein-like 3 Promotes Tumor Progression by Regulating Apoptosis and Angiogenesis in Colorectal Cancer
- PMID: 41151863
- PMCID: PMC12577601
- DOI: 10.21873/cgp.20553
Oxysterol-binding Protein-like 3 Promotes Tumor Progression by Regulating Apoptosis and Angiogenesis in Colorectal Cancer
Abstract
Background/aim: Oxysterol-binding protein-like 3 (OSBPL3) is a member of the intracellular lipid receptor and transporter protein family involved in regulating lipid metabolism. Altered OSBPL3 expression has been observed in various cancers, where it has been associated with both oncogenic and tumor-suppressive roles. However, its precise functions and underlying mechanisms in colorectal cancer (CRC) remain unclear. This study aimed to investigate the role of OSBPL3 in CRC cells and evaluate its prognostic significance.
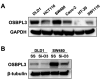
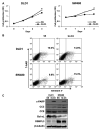
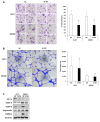
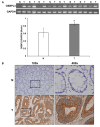
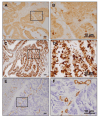
Materials and methods: A small interfering RNA vector targeting OSBPL3 was employed to silence its expression in CRC cell lines. OSBPL3 levels in CRC tissues were assessed using reverse transcription-polymerase chain reaction and immunohistochemistry. Tumor cell apoptosis, proliferation, and angiogenesis were evaluated via a terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling assay, and immunohistochemical staining for Ki-67 and CD34.
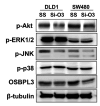
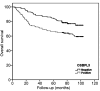
Results: OSBPL3 silencing inhibited tumor cell migration and invasion in CRC. OSBPL3 knockdown reduced proliferation and induced apoptosis through caspase activation and cell cycle arrest mediated by the regulation of cyclins, cyclin dependent kinases (CDKs), and CDK inhibitors. OSBPL3 suppression impaired the invasion and tube formation of human umbilical vein endothelial cells by down-regulating angiogenic factors and up-regulating angiostatic factors. OSBPL3 expression correlated significantly with tumor size, tumor stage, invasion depth, lymph node involvement, distant metastasis, and reduced survival. The apoptotic index was lower and microvessel density was higher in OSBPL3-positive tumors than in OSBPL3-negative tumors.
Conclusion: OSBPL3 contributes to CRC progression by regulating tumor cell apoptosis and angiogenesis.
Keywords: Oxysterol-binding protein-like 3; angiogenesis; apoptosis; colorectal neoplasm; prognosis.
Copyright © 2025, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare that they have no conflicts of interest in regard to this study.
Figures




References
-
- Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, Lim YC, Kibria KMK, Mohiuddin AKM, Ming LC, Goh KW, Hadi MA. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel) 2022;14(7):1732. doi: 10.3390/cancers14071732. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
