Tauroursodeoxycholic acid-induced increase in ectopic muscle mineralization occurs exclusively in dystrophic muscles and is independent of endoplasmic reticulum stress
- PMID: 41152473
- PMCID: PMC12569086
- DOI: 10.1038/s41598-025-21534-0
Tauroursodeoxycholic acid-induced increase in ectopic muscle mineralization occurs exclusively in dystrophic muscles and is independent of endoplasmic reticulum stress
Abstract
Calcification of dystrophic skeletal muscles was described previously and attributed, among others, to ER-stress, elevated phosphate concentration and chronic inflammation. Tauroursodeoxycholic acid (TUDCA) is considered an artificial chaperone protecting cells against ER-stress thus could prevent an ectopic mineralisation of soft tissues. Because an enhanced ER-stress is a feature of dystrophic muscles and it promotes soft tissue mineralisation we hypothesised that TUDCA treatment should reduce mineral deposits in dystrophic skeletal muscles, and tested this concept using two mouse models of DMD. Four-week old mdx, mdxβetageo and w/t mice were administered TUDCA in drinking water for 4 weeks. At 8 weeks, following tissue-clearing and calcium minerals staining with alizarin, mineralisation was evaluated using whole body scanning. Additionally, isolated skeletal muscles were analysed by Western blotting for ER-stress and calcification markers, and using various microscopic methods. Enzymatic activity of alkaline phosphatase was also assayed. Unexpectedly, TUDCA enhanced calcification of dystrophic but not dystrophin-positive muscles. TUDCA did not affect the elevated ER-stress markers found in dystrophic muscles nor impact pro-calcifying proteins RUNX2, Osterix and BMP2/4, which were also overexpressed in dystrophic muscles. The alkaline phosphatase levels, which were reduced in dystrophic muscles, were not affected by this treatment. The increase in ectopic calcification in dystrophic muscles induced by TUDCA is specific to muscles lacking dystrophin. This effect is not linked to the alleviation of ER stress or the overexpression of proteins directly involved in calcium mineral accumulation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval: Every effort was made to minimize the number of animals used and the level of stress they endure. All methods we used including anaesthesia and euthanasia were consistent with the commonly accepted norms of veterinary best practice. The study was carried out in accordance with relevant guidelines and regulations as described by the ARRIVE guidelines (PLoS Bio 8(6), e1000412, 2010). The experimental procedures also complied with the Polish Law on Experiments on Animals, which implements the European Council Directive, and were approved by the 1st Local Ethical Committee for Animal Experimentation in Warsaw (Permit Number: 1279/2022).
Figures



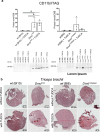

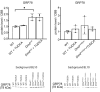
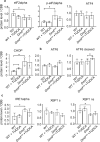
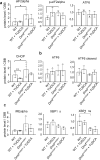



References
-
- Yiu, E. M. & Kornberg, A. J. Duchenne muscular dystrophy. J. Paediatr. Child Health51(8), 759–764. 10.1111/jpc.12868 (2015). - PubMed
-
- Snow, W. M., Anderson, J. E. & Jakobson, L. S. Neuropsychological and neurobehavioral functioning in Duchenne muscular dystrophy: a review. Neurosci Biobehav Rev.37(5), 743–752. 10.1016/j.neubiorev.2013.03.016 (2013). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

