Can We Successfully Discontinue Anti-Tumor Necrosis Factor-α Treatment in Children with Non-Systemic Juvenile Idiopathic Arthritis? The Experience of a Tertiary Center
- PMID: 41153616
- PMCID: PMC12561852
- DOI: 10.3390/biomedicines13102329
Can We Successfully Discontinue Anti-Tumor Necrosis Factor-α Treatment in Children with Non-Systemic Juvenile Idiopathic Arthritis? The Experience of a Tertiary Center
Abstract
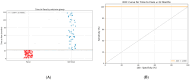
Background: Some patients with juvenile idiopathic arthritis (JIA) can successfully undergo withdrawal of treatment with anti-tumor necrosis factor alpha (anti-TNF-α) therapy, which may reduce economic and treatment-related burdens and the potential morbidity of treatment for at least 6 months. Currently, no guidelines exist on the appropriate withdrawal of anti-TNF-α therapy once clinically inactive disease (CID) has been achieved. This study aimed to assess the possibility of withdrawing anti-TNF-α therapy in children with non-systemic JIA after achieving long-term clinical remission. Methods: This single-center retrospective cohort study included data from 137 non-systemic JIA patients treated with anti-TNF-α therapy (etanercept or adalimumab) and having maintained CID for at least 24 months during treatment. Demographic, laboratory, and treatment data were collected at JIA onset, at the initiation of anti-TNF-α therapy, every 6 months during therapy, and at the time of disease flare. Anti-TNF-α therapy was discontinued abruptly after discussing it with the patients and their families in each case. Outcomes were assessed using standard criteria for remission in JIA (ACRpedi and Wallace criteria). Results: Following withdrawal of TNF-α inhibitors, 93/137 patients (67.9%) experienced a disease flare, with a median time to flare of 7 months (3; 14). Thirty-two percent of patients remained in remission for a median of 63 months. Absence of flare during the first 22 months after discontinuation was associated with prolonged biologic-free remission (odds ratio 682; 95% CI 38.6-12,062; p < 0.0001), with 83% sensitivity and 100% specificity (area under the ROC curve 0.967). Most flares involved arthritis (76%) and/or uveitis (24%), primarily affecting knees, ankles, and wrists. Inflammatory markers were generally lower at flare compared to baseline. Biological therapy was resumed in 84/93 patients (90.3%), achieving at least a 50% improvement according to ACRpedi criteria within 3 months and remission according to Wallace criteria within 6 months. Conclusion: Over two-thirds of patients with non-systemic JIA who achieve CID experience a flare within seven months of anti-TNF-α discontinuation. Re-initiation of biologic therapy is effective in restoring remission. These results indicate that prolonged biologic-free remission is possible in a subset of patients, highlighting the need for individualized withdrawal strategies and careful post-discontinuation monitoring.
Keywords: anti-TNF-α; biologic; clinical remission; disease activity; etanercept; juvenile idiopathic arthritis; medicine tapering; outcomes.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


References
-
- Petty R.E., Southwood T.R., Manners P., Baum J., Glass D.N., Goldenberg J., He X., Maldonado-Cocco J., Orozco-Alcala J., Prieur A.M., et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: Second revision, Edmonton, 2001. J. Rheumatol. 2004;31:390–392. - PubMed
-
- Lovell D.J., Giannini E.H., Reiff A., Jones O.Y., Schneider R., Olson J.C., Stein L.D., Gedalia A., Ilowite N.T., Wallace C.A., et al. Long-term efficacy and safety of etanercept in children with polyarticular-course juvenile rheumatoid arthritis. Arthritis Rheum. 2003;48:218–226. doi: 10.1002/art.10710. - DOI - PubMed
-
- Bongartz T., Sutton A.J., Sweeting M.J., Buchan I., Matteson E.L., Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: Systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA. 2006;295:2275–2285. doi: 10.1001/jama.295.19.2275. - DOI - PubMed
-
- Tynjälä P., Vähäsalo P., Tarkiainen M., Kröger L., Aalto K., Malin M., Putto-Laurila A., Honkanen V., Lahdenne P. Aggressive combination drug therapy in very early polyarticular juvenile idiopathic arthritis (ACUTE-JIA): A multicentre randomized open-label clinical trial. Ann. Rheum. Dis. 2011;70:1605–1612. doi: 10.1136/ard.2010.143347. - DOI - PubMed
LinkOut - more resources
Full Text Sources

