Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis
- PMID: 41154401
- PMCID: PMC12562807
- DOI: 10.3390/cancers17203346
Hepatocellular Carcinoma Growth Kinetics and Outcomes After Transarterial Embolization: A Single-Center Analysis
Abstract
Purpose: This study examines the association between tumor volume doubling time (TVDT) and clinical outcomes for patients with hepatocellular carcinoma (HCC) treated with transarterial embolization (TAE) and evaluates the impact of tumor genotype on TVDT.
Methods: This was a retrospective cohort study at a single tertiary care cancer center, including treatment-naïve patients with biopsy-proven HCC treated with TAE from 1/2014 to 6/2022. The patients underwent initial baseline contrast-enhanced cross-sectional imaging more than 30 days prior to embolization. Index tumors were defined as the largest HCC present on baseline imaging treated with TAE, and TVDT was calculated using Schwartz's equation with perpendicular trans-axial measurements. Genetic mutation analysis was performed on HCC tissue specimens using next-generation sequencing. Survival analysis was performed using the Kaplan-Meier method, and Cox regression was used to assess prognostic factors for survival.
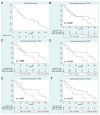
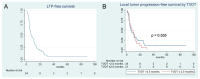
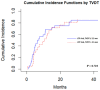
Results: Patients with a TVDT ≤ 2.5 months had a worse overall survival (OS) after TAE (p = 0.011). On multivariate regression analysis, predictors of worse OS following TAE included TVDT ≤ 2.5 months (p = 0.036), Eastern Cooperative Oncology Group (ECOG) performance score of 1 or 2 (p = 0.006), and alpha fetoprotein > 200 ng/mL (p = 0.013). The presence of ≥5 HCC tumors on initial imaging was associated with a worse local tumor progression-free survival (LTPFS) on multivariate analysis (p = 0.002). No single genetic mutation was associated with shorter TVDT.
Conclusion: Patients with HCC exhibiting rapid growth, defined as shorter TVDT, may be associated with worse overall survival following TAE. Rapid tumor growth does not seem to be correlated with a single genetic mutation.
Keywords: growth kinetics; hepatocellular carcinoma; transarterial embolization.
Conflict of interest statement
Dr. Zhao has received a research grant from the SIR Foundation. Dr. Erinjeri is a consultant for AstraZeneca. Dr. Alexander is a consultant for Boston Scientific. Dr. Sotirchos is a consultant for Intera Medical. Dr. Harding has received research support from NCI P30-CA008748, NCI U01 CA238444 04, the Society of Memorial Sloan Kettering Cancer Center, Experimental Therapeutics Center, Cycle for Survival, AbbVie, Bristol Myers Squibb, Boehringer Ingelheim, CytomX, Debiopharm, Eli Lilly, Genoscience, Incyte, Kinnate Biopharma, Loxo @ Lilly, Novartis, Polaris, Pfizer, Tvardi, Zymeworks, and Yiviva. Dr. Harding is a consultant for Adaptimmune, AstraZeneca, Bristol Myers Squibb, Exelexis, Elevar, Eisai, Genoscience (uncompensated), Hepion, Imvax, Merck (DSMB), Medivir, QED, RayzeBio, Servier, Tempus, Tyra, and Zymeworks (uncompensated). Dr. Ziv has received research grants from MSK, Druckenmiller, NETRF, AACR, NANETS, SIR, RSNA, Ethicon, Novartis, and ALA. Dr. Covey is a stockholder of Amgen, a member of the advisory board of Boston Scientific, a paid speaker for Oncology Live, and a section editor of AJR. Dr. Sofocleous has received research support from the NCI/NIH, SIO, SIR Foundation, Boston Scientific, MIM, SIRTEX, Johnson and Johnson, and VARIAN. Dr. Sofocleous is a consultant or member of the advisory board for Terumo, Varian, SIRTEX, and Johnson and Johnson. Dr. Sofocleous has served as a member of the Board of Directors of SIO, 2017–2023, and is currently a member of the SIR executive council. Dr. Yarmohammadi has received research grants from the Thompson Foundation and Guerbet. The remaining authors declare no conflict of interest. The funders had no role in the design of this study; in the collection, analyses, or interpretation of data; in the writing of this manuscript; or in the decision to publish the results.
Figures


References
-
- Reig M., Forner A., Rimola J., Ferrer-Fabrega J., Burrel M., Garcia-Criado A., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. - DOI - PMC - PubMed
-
- Benson A.B., D’Angelica M.I., Abbott D.E., Anaya D.A., Anders R., Are C., Bachini M., Borad M., Brown D., Burgoyne A., et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021;19:541–565. doi: 10.6004/jnccn.2021.0022. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

