The Redox Paradox: Cancer's Double-Edged Sword for Malignancy and Therapy
- PMID: 41154495
- PMCID: PMC12561025
- DOI: 10.3390/antiox14101187
The Redox Paradox: Cancer's Double-Edged Sword for Malignancy and Therapy
Abstract
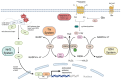
Reactive oxygen species (ROS) function as critical signaling molecules in cancer biology, promoting proliferation, angiogenesis, and metastasis at controlled levels while inducing lethal damage when exceeding the cell's buffering capacity. To survive under this state of chronic oxidative stress, cancer cells become dependent on a hyperactive antioxidant shield, primarily orchestrated by the Nrf2, glutathione (GSH), and thioredoxin (Trx) systems. These defenses maintain redox homeostasis and sustain oncogenic signaling, notably through the oxidative inactivation of tumor-suppressor phosphatases, such as PTEN, which drives the PI3K/AKT/mTOR pathway. Targeting this addiction to a rewired redox state has emerged as a compelling therapeutic strategy. Pro-oxidant therapies aim to overwhelm cellular defenses, with agents like high-dose vitamin C and arsenic trioxide (ATO) showing significant tumor-selective toxicity. Inhibiting the master regulator Nrf2 with compounds such as Brusatol or ML385 disrupts the core antioxidant response. Disruption of the GSH system by inhibiting cysteine uptake with sulfasalazine or erastin potently induces ferroptosis, a non-apoptotic cell death driven by lipid peroxidation. Furthermore, the thioredoxin system is targeted by the repurposed drug auranofin, which irreversibly inhibits thioredoxin reductase (TrxR). Extensive preclinical data and ongoing clinical trials support the concept that this reliance on redox adaptation is a cancer-selective vulnerability. Moreover, novel therapeutic strategies, including the expanding field of redox-active metal complexes, such as manganese porphyrins, which strategically leverage the differential redox state of normal versus cancer cells through both pro-oxidant and indirect Nrf2-mediated antioxidative mechanisms (triggered by Keap1 oxidation), with several agents currently in advanced clinical trials, have also been discussed. Essentially, pharmacologically tipping the redox balance beyond the threshold of tolerance offers a rational and powerful approach to eliminate malignant cells, defining a novel frontier for targeted cancer therapy.
Keywords: Nrf2; PI3K/AKT/Mtor; PTEN; cancer; cancer therapy; ferroptosis; glutathione (GSH); oxidative stress; reactive oxygen species (ROS); redox signaling; thioredoxin (Trx); tumor microenvironment (TME).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Chen C., Mann G.E. Discussion on current topics in redox biology and future directions/ year in review. Free Radic. Biol. Med. 2025;233:S18. doi: 10.1016/j.freeradbiomed.2025.05.069. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

