Combined XPO1 Inhibition and Parthenolide Treatment Can Be Efficacious in Treating Triple-Negative Breast Cancer
- PMID: 41155531
- PMCID: PMC12564529
- DOI: 10.3390/ijms262010243
Combined XPO1 Inhibition and Parthenolide Treatment Can Be Efficacious in Treating Triple-Negative Breast Cancer
Abstract
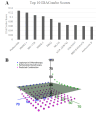
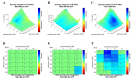
Triple-negative breast cancer (TNBC) is an aggressive, heterogeneous subtype of breast cancer with limited treatment options. Our previous work explored repurposing selinexor, an XPO1 inhibitor, as a novel therapeutic option for TNBC. To enhance its efficacy, this study aimed to identify beneficial combination therapies with selinexor and experimentally evaluate their effects in TNBC. Using the computational tool IDACombo, we nominated drugs predicted to improve the efficacy of XPO1 inhibition. The top candidate, parthenolide, was tested in vitro using three transcriptionally distinct TNBC cell lines. Fluorescently labeled cells were co-cultured and treated with selinexor, parthenolide, or their combination. Growth inhibition was assessed across the mixed population and by individual cell line after 96 h, and potential synergy was evaluated using Combenefit. While selinexor and parthenolide monotherapy inhibited the growth of TNBC subtypes, the combination was more effective in suppressing the overall cell population. Synergistic interactions between the two agents were observed in specific TNBC lines but not all, reflecting the combination effect in heterogeneous TNBC patients. Our findings suggest the selinexor-parthenolide combination as a potential therapeutic strategy for TNBC, warranting further investigation. Our study also demonstrates the value of integrative computational-experimental approaches in guiding heterogeneity-informed drug combinations for preclinical evaluation.
Keywords: NFKBIA; XPO1; combination therapy; parthenolide; selective inhibitors of nuclear export (SINEs); triple negative breast cancer (TNBC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

