Innovative Amino-Functionalization of Pyrido[2,3- d]pyrimidine Scaffolds for Broad Therapeutic Applications Supported by Computational Analyses
- PMID: 41155588
- PMCID: PMC12567244
- DOI: 10.3390/ph18101472
Innovative Amino-Functionalization of Pyrido[2,3- d]pyrimidine Scaffolds for Broad Therapeutic Applications Supported by Computational Analyses
Abstract
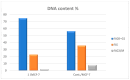
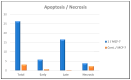
Background: Derivatives of Pyrido[2,3-d]pyrimidine-6-carboxylate are promising multi-target scaffolds. This study focused on synthesizing 16 amino-functionalized derivatives and evaluating their dual anticancer and antibacterial activities, supported by mechanistic and computational analyses. Objectives: Design and synthesize derivatives, evaluate cytotoxicity against HeLa, HepG-2, and MCF-7 (selectivity against WI-38), investigate EGFRWT and EGFRT790M inhibition, assess cell cycle, apoptosis, and migration effects, antibacterial efficacy against E. coli and P. aeruginosa, and perform in silico ADMET, docking, molecular dynamics, DFT, and antiviral predictions. Methods: Synthesized 16 derivatives; tested for cytotoxicity, EGFR inhibition, cell cycle, apoptosis, migration; assessed antibacterial activity; performed ADMET profiling, molecular docking, molecular dynamics, and DFT calculations. Results: Derivatives 1, 2, and 7 showed highest cytotoxicity (IC50 = 3.98-17.52 μM; WI-38 IC50 = 64.07-81.65 μM). Compound 1 potently inhibited EGFRWT (IC50 = 0.093 μM) and EGFRT790M (IC50 = 0.174 μM), induced G0/G1 arrest (74.86%) and apoptosis (26.37%), and reduced MCF-7 migration (69.63%). Moderate antibacterial activity observed (MIC = 50 μg/mL). ADMET indicated favorable pharmacokinetics, low CYP inhibition, negative mutagenicity, and oral toxicity class III. Molecular dynamics confirmed stable binding (EGFRWT RMSD 3 Å; EGFRT790M 3.5-4.6 Å) with persistent hydrogen bonds. In silico antiviral evaluation suggested strong binding to HCV NS5A (-9.36 kcal/mol), SARS-CoV-2 Mpro (-9.82 kcal/mol), and E.coli DNA gyrase (-10.25 kcal/mol). Conclusions: Compound 1 exhibits dual anticancer and antibacterial activity, supported by mechanistic and computational analyses, highlighting pyrido[2,3-d]pyrimidines as promising multi-target therapeutic scaffolds.
Keywords: DFT; EGFR inhibition; antibacterial activity; antiviral prediction; apoptosis; molecular docking; molecular dynamics; pyridopyrimidine derivatives; wound healing assay.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


























References
-
- Bizuayehu H.M., Ahmed K.Y., Kibret G.D., Dadi A.F., Belachew S.A., Bagade T., Tegegne T.K., Venchiarutti R.L., Kibret K.T., Hailegebireal A.H., et al. Global disparities of cancer and its projected burden in 2050. JAMA Netw. Open. 2024;7:e2443198. doi: 10.1001/jamanetworkopen.2024.43198. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

