Extracellular Vesicles and Nanoparticles in Regenerative and Personalised Medicine: Diagnostic and Therapeutic Roles-A Narrative Review
- PMID: 41155965
- PMCID: PMC12566876
- DOI: 10.3390/pharmaceutics17101331
Extracellular Vesicles and Nanoparticles in Regenerative and Personalised Medicine: Diagnostic and Therapeutic Roles-A Narrative Review
Abstract
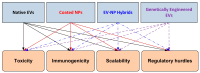
Background: Degenerative, metabolic and oncologic diseases are scarcely amenable to the complete reconstruction of tissue structure and functionalities using common therapeutic modalities. On the nanoscale, extracellular vesicles (EVs) and nanoparticles (NPs) have emerged as attractive candidates in regenerative and personalised medicine. However, EV transfection is hindered by its heterogeneity and low yield, while NPs suffer from cytotoxicity, immunogenicity, and long-term safety issues. Scope of Review: This review synthesises data from over 180 studies as part of a narrative synthesis, critically evaluating the disease-specific utility, mechanistic insights, and translational obstacles. The focus is laid on comparative cytotoxicity profiles, the capacities of hybrid EV-NP systems to circumvent mutual shortcomings, and the increasing impact of artificial intelligence (AI) on predictive modelling, as well as toxicity appraisal and manufacturing. Key Insights: EVs have inherent biocompatibility, immune evasive and organotropic signalling functions; NPs present structural flexibility, adjustable physicochemical properties, and industrial scalability. Common molecular pathways for NP toxicity, such as ROS production, MAPK and JAK/STAT activation, autophagy, and apoptosis, are significant biomarkers for regulatory platforms. Nanotechnological and biomimetic nanocarriers incorporate biological tropism with engineering control to enhance therapeutic efficacy, as well as their translational potential. AI approaches can support rational drug design, promote reproducibility across laboratories, and meet safe-by-design requirements. Conclusions: The intersection of EVs, NPs and AI signifies a turning point in regenerative nanomedicine. To advance this field, there is a need for convergence on experimental protocols, the adoption of mechanistic biomarkers, and regulatory alignment to ensure reproducibility and clinical competence. If realised, these endeavours will not only transition nanoscale medicament design from experimental constructs into reliable and patient-specific tools for clinical trials, but we also have the strong expectation that they could revolutionise future treatments of challenging human disorders.
Keywords: artificial intelligence; cytotoxicity; extracellular vesicles; hybrid nanoplatforms; nanoparticles; personalised medicine; regenerative medicine; translational barriers.
Conflict of interest statement
Author Bogdan Tudora was employed by the company Sigma Evolution. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

