Design, Synthesis and Biological Evaluation of Pyrazolopyrimidine Derivatives as Aryl Hydrocarbon Receptor Antagonists for Colorectal Cancer Immunotherapy
- PMID: 41155994
- PMCID: PMC12567141
- DOI: 10.3390/pharmaceutics17101359
Design, Synthesis and Biological Evaluation of Pyrazolopyrimidine Derivatives as Aryl Hydrocarbon Receptor Antagonists for Colorectal Cancer Immunotherapy
Abstract
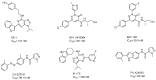
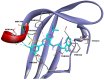
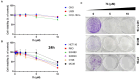
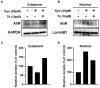
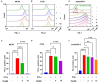
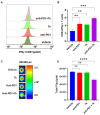
Background: Aryl hydrocarbon receptor (AhR) is a transcription factor that is involved in the regulation of immunity. AhR inhibits T cell activation in tumors, which induces immune suppression in the blood and solid tumors. We identified effective small-molecule AhR antagonists for cancer immunotherapy. Methods: A new series of pyrazolopyrimidine derivatives was synthesized and evaluated for AhR antagonistic activity. Results: Compound 7k exhibited significant antagonistic activity against AhR in a transgenic zebrafish model. In addition, 7k exhibited good AhR antagonist activity, with a half-maximal inhibitory concentration (IC50) of 13.72 nM. Compound 7k showed a good pharmacokinetic profile with an oral bioavailability of 71.0% and a reasonable half-life of 3.77 h. Compound 7k selectively exerted anti-proliferative effects on colorectal cancer cells without affecting normal cells, concurrently suppressing the expression of AhR-related genes and the PD-1/PD-L1 signaling pathway. Compound 7k exhibited potent antitumor activity in syngeneic colorectal cancer models. Importantly, the combination of anti-PD1 and compound 7k enhanced antitumor immunity by augmenting cytotoxic T lymphocyte (CTL)-mediated activity. Conclusions: Collectively, a new pyrazolopyrimidine derivative, 7k, shows promise as a potential therapeutic agent for treating colorectal cancer.
Keywords: antagonist; aryl hydrocarbon receptor; cancer; zebrafish.
Conflict of interest statement
Prof. Dr. Jin Hee Ahn was employed by JD Bioscience. Dr. Yong Hyun Jeon, Jae-Eon Lee, So Yeon Jeong, and Geumi Park were affiliated with the Preclinical Research Center, Daegu-Gyeongbuk Medical Innovation Foundation (K-MEDIhub). Dr. Kyoung-jin Min and Dr. Heegyum Moon were affiliated with the New Drug Development Center, K-MEDIhub. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures










References
-
- Karasová M., Procházková J., Tylichová Z., Fedr R., Ciganek M., Machala M., Dvořák Z., Vyhlídalová B., Zůvalová I., Ehrmann J., et al. Inhibition of Aryl Hydrocarbon Receptor (AhR) Expression Disrupts Cell Proliferation and Alters Energy Metabolism and Fatty Acid Synthesis in Colon Cancer Cells. Cancers. 2022;14:4245. doi: 10.3390/cancers14174245. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

