Therapeutic Modulation of the Gut Microbiome by Supplementation with Probiotics (SCI Microbiome Mix) in Adults with Functional Bowel Disorders: A Randomized, Double-Blind, Placebo-Controlled Trial
- PMID: 41156743
- PMCID: PMC12566429
- DOI: 10.3390/microorganisms13102283
Therapeutic Modulation of the Gut Microbiome by Supplementation with Probiotics (SCI Microbiome Mix) in Adults with Functional Bowel Disorders: A Randomized, Double-Blind, Placebo-Controlled Trial
Abstract
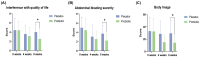
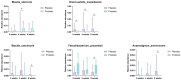
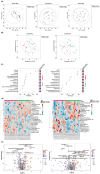
Functional bowel disorders (FBDs) are chronic gastrointestinal conditions characterized by recurrent symptoms associated with gut microbiota dysbiosis. Although accumulating evidence suggests that probiotics can improve symptoms in patients with FBD, the underlying mechanisms remain to be fully elucidated. In this randomized, double-blind, placebo-controlled clinical trial, 38 adults meeting the Rome IV diagnostic criteria of functional constipation (FC) and functional diarrhea (FD) received either a multi-strain probiotic complex or placebo for 8 weeks. Clinical outcomes were evaluated using the Irritable Bowel Syndrome Severity Scoring System (IBS-SSS), bowel habits questionnaire, and IBS Quality of Life (IBS-QoL) instrument. Fecal samples were collected at baseline and at week 8 for gut microbiota profiling via 16S rRNA gene sequencing and metabolomic analysis using gas chromatography-mass spectrometry. Probiotic supplementation significantly reduced the severity of abdominal bloating and its interference with quality of life, and improved the body image domain of the IBS-QoL. Beta diversity analysis showed significant temporal shifts in the probiotic group, while 16S rRNA sequencing revealed an increased relative abundance of Faecalibacterium prausnitzii and Blautia stercoris. Fecal metabolomic analysis further indicated elevated levels of metabolites implicated in the gut-brain axis. Multi-strain probiotic supplementation alleviated gastrointestinal symptoms and improved aspects of psychosocial well-being in adults with FBDs, potentially through modulation of the human gut microbiome.
Keywords: Blautia stercoris; Faecalibacterium prausnitzii; clinical trial; functional bowel disorders; human gut microbiome; probiotics; serotonin.
Conflict of interest statement
W.Y.B., H.K., H.B.L., and J.S.M. were employed by the company ILDONG Bioscience. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources

